SUMMARY: The FDA on December 8, 2015 allowed the marketing of the first cooling cap in the US, Dignitana DigniCap Cooling System, to reduce hair loss, in female breast cancer patients undergoing chemotherapy. Alopecia (hair loss) is a common side effect of several chemotherapeutic agents and can be emotionally traumatic. Even though temporary, minimizing or alleviating hair loss, can have a significant impact on patients psychological well being and willingness to pursue necessary treatment. Presently available non-FDA approved cooling devices include Penguin Cold Caps and Paxman Scalp Cooling System, although the later is not available in the US. One of the major concerns with cold caps use has been the risk for scalp metastasis due to decreased chemotherapy access to the scalp tissue from vasoconstriction associated with cooling devices. It is clear now that that the risk of metastases to the scalp is extremely rare and low (1.2%) and even lower as an initial event for advanced disease.
The Dignitana DigniCap computer-controlled cooling system pumps liquid coolant through a head-worn silicone cooling cap during chemotherapy treatment. This cooling cap is covered by an outer insulating cap which holds the cooling cap in place. The circulating coolant inside the cap gradually gets colder. The cold and near freezing temperature constricts the blood vessels in the scalp, which, in turn reduces chemotherapy access in the hair follicles, as well as metabolic activity of the hair follicle cells, thus slowing cell division. This combined action impairs the effect of chemotherapy on hair follicles and reduces chemotherapy induced hair loss.
The FDA approval was based on a multicenter prospective open-label, nonrandomized study in which the efficacy of the cooling system was studied in 122 women with Stage I and Stage II breast cancer who were receiving chemotherapy regimens associated with hair loss. The primary endpoint was patient self-assessment of hair loss using standardized photographs at three to six weeks after the last chemotherapy cycle. A score of 0-2 (50% or less hair loss) was defined as treatment success. Patients who chose not to undergo scalp cooling were enrolled in a control group. It was noted that more than 66 percent of patients treated with the DigniCap reported losing less than half their hair whereas 94% had more than 75% hair loss in the control group. The most common side effects with the scalp cooling system included cold-induced headaches and neck and shoulder discomfort, chills and pain associated with wearing the cooling cap for prolonged period of time.
The authors concluded that the DigniCap System is highly effective in reducing chemotherapy-induced alopecia and the FDA approval of this scientifically proven option will provide a major relief for cancer patients receiving chemotherapy. Clinical performance of the DigniCap system, a scalp hypothermia system, in preventing chemotherapy-induced alopecia. Rugo HS, Klein P, Melin SA, et al. J Clin Oncol 33, 2015 (suppl; abstr 9518)

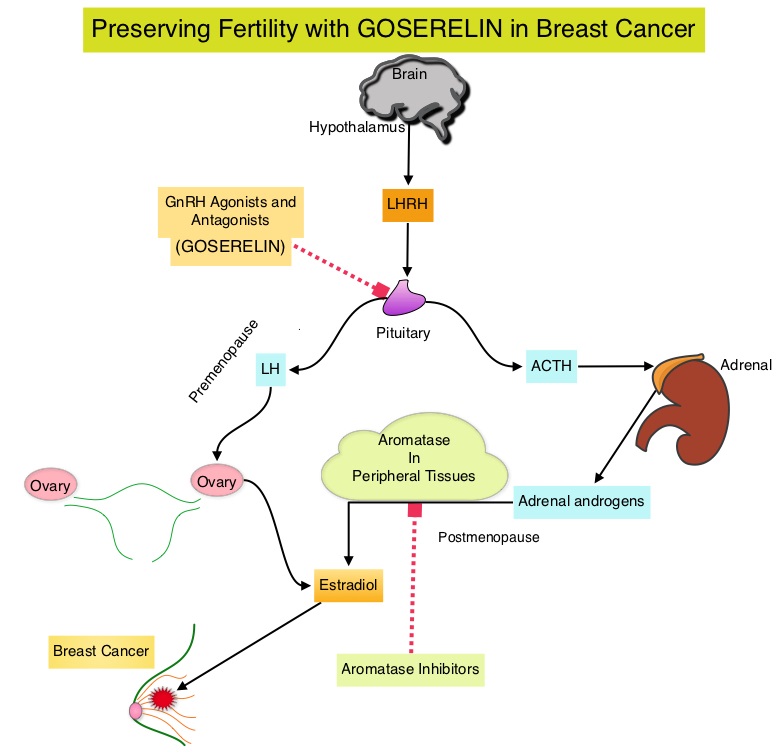
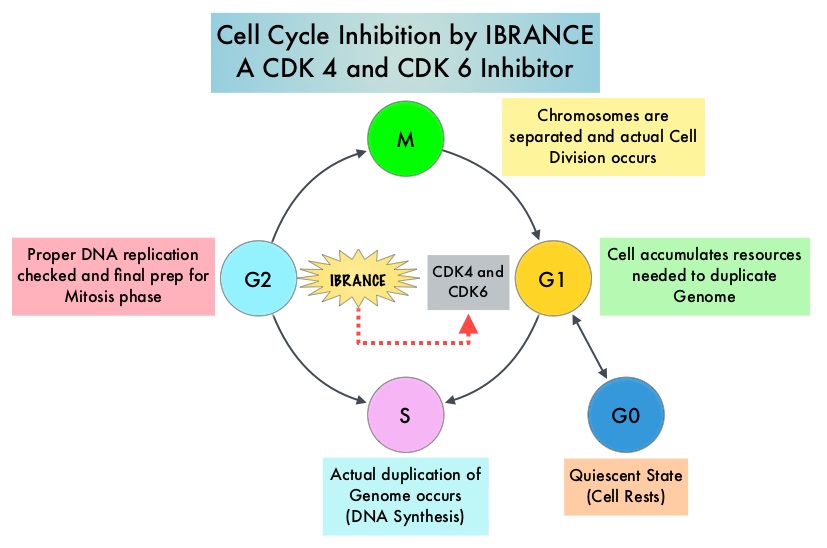 Approximately 80% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB) and initiate transition from the G1 phase to the S phase of the cell cycle. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. IBRANCE® (Palbociclib) is a reversible, oral, selective, small molecule inhibitor of Cyclin Dependent Kinases, CDK4 and CDK6, and prevent RB1 phosphorylation. IBRANCE® is the first CDK inhibitor approved by the FDA. It exhibits synergy when combined with endocrine therapies. In an open-label, randomized, phase II study, which included treatment naïve postmenopausal women with ER-positive, HER2-negative, advanced breast cancer, IBRANCE® given along with Aromatase Inhibitor FEMARA® (Letrozole) significantly prolonged Progression Free Survival, Overall Response rate and median duration of response, compared to FEMARA® alone. Based on this data, the U. S. Food and Drug Administration on February 3, 2015 granted accelerated approval to IBRANCE® (Palbociclib), for use in combination with FEMARA® (Letrozole) in this patient population. FASLODEX® (Fulvestrant) is a selective estrogen receptor down-regulator presently indicated for the treatment of hormone receptor positive metastatic breast cancer patients, with disease progression following antiestrogen therapy.
Approximately 80% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB) and initiate transition from the G1 phase to the S phase of the cell cycle. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. IBRANCE® (Palbociclib) is a reversible, oral, selective, small molecule inhibitor of Cyclin Dependent Kinases, CDK4 and CDK6, and prevent RB1 phosphorylation. IBRANCE® is the first CDK inhibitor approved by the FDA. It exhibits synergy when combined with endocrine therapies. In an open-label, randomized, phase II study, which included treatment naïve postmenopausal women with ER-positive, HER2-negative, advanced breast cancer, IBRANCE® given along with Aromatase Inhibitor FEMARA® (Letrozole) significantly prolonged Progression Free Survival, Overall Response rate and median duration of response, compared to FEMARA® alone. Based on this data, the U. S. Food and Drug Administration on February 3, 2015 granted accelerated approval to IBRANCE® (Palbociclib), for use in combination with FEMARA® (Letrozole) in this patient population. FASLODEX® (Fulvestrant) is a selective estrogen receptor down-regulator presently indicated for the treatment of hormone receptor positive metastatic breast cancer patients, with disease progression following antiestrogen therapy.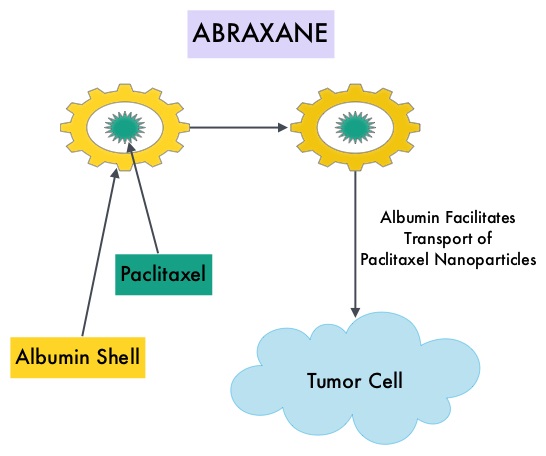 ABRAXANE® (Nab-paclitaxel) is a solvent free, albumin-encapsulated nanoparticle formulation of the taxane, Paclitaxel. By virtue of its formulation, unlike TAXOL®, hypersensitivity reactions are uncommon with ABRAXANE® and can therefore be rapidly infused and premedications are not needed. Further, higher tumor drug concentrations are achieved with ABRAXANE® compared to conventional Paclitaxel (TAXOL®). Previously published studies have shown that ABRAXANE® is superior to TAXOL® in metastatic breast cancer. GeparSepto trial is a randomized phase III study in which weekly ABRAXANE® was compared head to head with weekly TAXOL® in a neoadjuvant setting. This study enrolled 1204 treatment naïve, high risk patients, with clinical T2-T4d invasive breast carcinoma. Eligible patients included those with unilateral, bilateral, operable or inoperable breast cancer. The median age was 49 years, median tumor size was 3 cm, 23% of the patients had triple-negative disease and 33% had HER-2 positive tumors. Patients were randomized 1:1 to receive either ABRAXANE® 125 mg/m2 IV or TAXOL® 80 mg/m2 IV, weekly for 12 weeks followed by 4 cycles of Epirubicin 90 mg/m2 IV and Cyclophosphamide 600 mg/m2 IV. Patients with HER-2 positive tumors also received HERCEPTIN® (Trastuzumab) and PERJETA® (Pertuzumab). The primary endpoint was pathologic Complete Response (pCR), defined as absence of microscopic residual invasive or noninvasive viable tumor cells in all resected specimens of the breast and axilla. This endpoint was chosen because pathologic Complete Response serves as a surrogate marker for long-term efficacy and outcomes. In this trial, the researchers noted a pathologic Complete Response rate of 38% with ABRAXANE® compared to 29% with TAXOL® (P<0.01). On subgroup analysis, this benefit was even more evident in patients with triple negative breast cancer (N=275), with a pCR rate of 48.2% in the ABRAXANE® group compared with 25.7% in the TAXOL® group (P <0.001). The incidence of peripheral neuropathy was higher in the ABRAXANE® group compared to TAXOL® group and this was attributed to higher weekly doses of ABRAXANE® administered. It is felt that a lower dose of weekly ABRAXANE® (100 mg/m2) would result in a decrease in the incidence of peripheral neuropathy, without compromising efficacy. The authors concluded that ABRAXANE® is superior to TAXOL® in early stage, high risk patients with breast cancer and this benefit is even more evident in those patients with triple negative disease, which comprises about 15% of all breast cancers. Untch M, Jackisch C, Schneeweiss A, et al. A randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69. Paper presented at: 2014 San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. Abstract S2-07.
ABRAXANE® (Nab-paclitaxel) is a solvent free, albumin-encapsulated nanoparticle formulation of the taxane, Paclitaxel. By virtue of its formulation, unlike TAXOL®, hypersensitivity reactions are uncommon with ABRAXANE® and can therefore be rapidly infused and premedications are not needed. Further, higher tumor drug concentrations are achieved with ABRAXANE® compared to conventional Paclitaxel (TAXOL®). Previously published studies have shown that ABRAXANE® is superior to TAXOL® in metastatic breast cancer. GeparSepto trial is a randomized phase III study in which weekly ABRAXANE® was compared head to head with weekly TAXOL® in a neoadjuvant setting. This study enrolled 1204 treatment naïve, high risk patients, with clinical T2-T4d invasive breast carcinoma. Eligible patients included those with unilateral, bilateral, operable or inoperable breast cancer. The median age was 49 years, median tumor size was 3 cm, 23% of the patients had triple-negative disease and 33% had HER-2 positive tumors. Patients were randomized 1:1 to receive either ABRAXANE® 125 mg/m2 IV or TAXOL® 80 mg/m2 IV, weekly for 12 weeks followed by 4 cycles of Epirubicin 90 mg/m2 IV and Cyclophosphamide 600 mg/m2 IV. Patients with HER-2 positive tumors also received HERCEPTIN® (Trastuzumab) and PERJETA® (Pertuzumab). The primary endpoint was pathologic Complete Response (pCR), defined as absence of microscopic residual invasive or noninvasive viable tumor cells in all resected specimens of the breast and axilla. This endpoint was chosen because pathologic Complete Response serves as a surrogate marker for long-term efficacy and outcomes. In this trial, the researchers noted a pathologic Complete Response rate of 38% with ABRAXANE® compared to 29% with TAXOL® (P<0.01). On subgroup analysis, this benefit was even more evident in patients with triple negative breast cancer (N=275), with a pCR rate of 48.2% in the ABRAXANE® group compared with 25.7% in the TAXOL® group (P <0.001). The incidence of peripheral neuropathy was higher in the ABRAXANE® group compared to TAXOL® group and this was attributed to higher weekly doses of ABRAXANE® administered. It is felt that a lower dose of weekly ABRAXANE® (100 mg/m2) would result in a decrease in the incidence of peripheral neuropathy, without compromising efficacy. The authors concluded that ABRAXANE® is superior to TAXOL® in early stage, high risk patients with breast cancer and this benefit is even more evident in those patients with triple negative disease, which comprises about 15% of all breast cancers. Untch M, Jackisch C, Schneeweiss A, et al. A randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69. Paper presented at: 2014 San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. Abstract S2-07.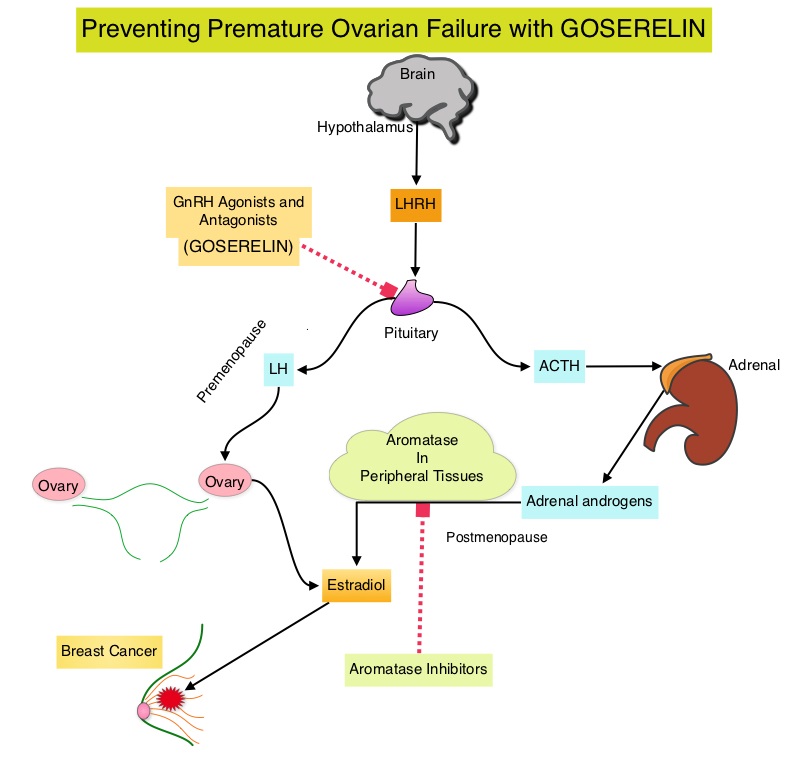 Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone receptor negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. The median age of the patients was 38 years and median follow up was 4.1 years. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.04). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of Premature Ovarian Failure with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy. Moore HC, Unger JM, Phillips K, et al. N Engl J Med 2015; 372:923-932
Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone receptor negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. The median age of the patients was 38 years and median follow up was 4.1 years. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.04). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of Premature Ovarian Failure with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy. Moore HC, Unger JM, Phillips K, et al. N Engl J Med 2015; 372:923-932
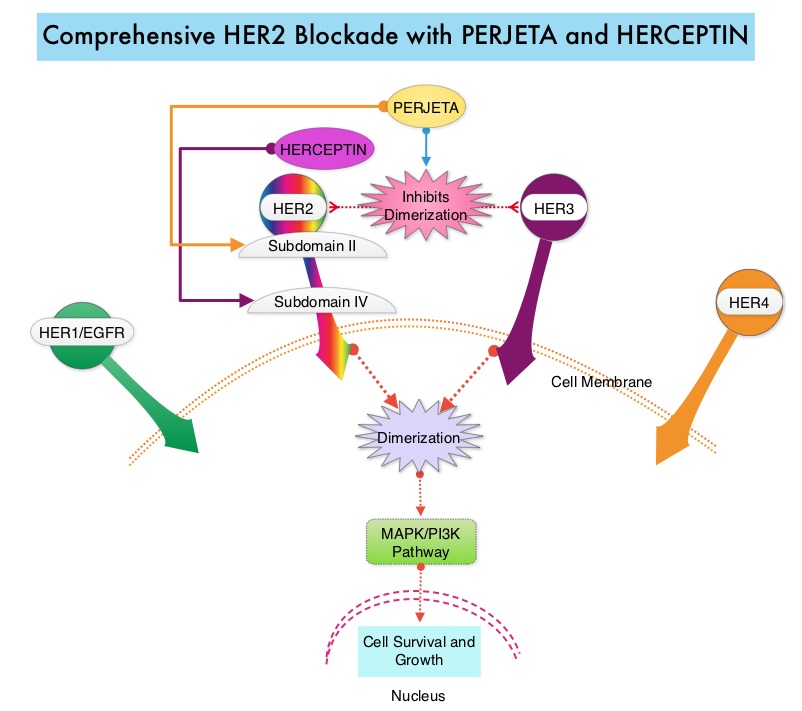 PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P<0.001). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001), at the time of the final analysis. The median duration of response was 20.2 months with the PERJETA® combination compared to 12.5 months in the non-PERJETA® group. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Baselga J, Kim S, et al. N Engl J Med 2015; 372:724-734
PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P<0.001). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001), at the time of the final analysis. The median duration of response was 20.2 months with the PERJETA® combination compared to 12.5 months in the non-PERJETA® group. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Baselga J, Kim S, et al. N Engl J Med 2015; 372:724-734