SUMMARY: It is estimated that in the US, approximately 96,480 new cases of Melanoma will be diagnosed in 2019 and about 7,230 patients are expected to die of the disease. The incidence of Melanoma has been on the rise for the past three decades. Surgical resection with a curative intent is the standard of care for patients with early stage Melanoma, with a 5-year survival rate of 98% for stage I disease and 90% for stage II disease. Patients with locally advanced or metastatic Melanoma historically have had poor outcomes. With the development and availability of immune checkpoint inhibitors and BRAF and MEK inhibitors, this patient group now has significantly improved outcomes. In treatment naïve patients receiving anti-PD-1 therapies such as KEYTRUDA® (Pembrolizumab) or OPDIVO® (Nivolumab) in phase 3 trials, the Progression Free Survival (PFS) rates have ranged from 27-31%, with an Overall Survival (OS) rate of 46% at 4 years. The 5-year OS among patients receiving KEYTRUDA® was 43%, and in those treated with a combination of OPDIVO® plus YERVOY® (Ipilimumab), 4-year PFS and OS rates were 37% and 53%, respectively.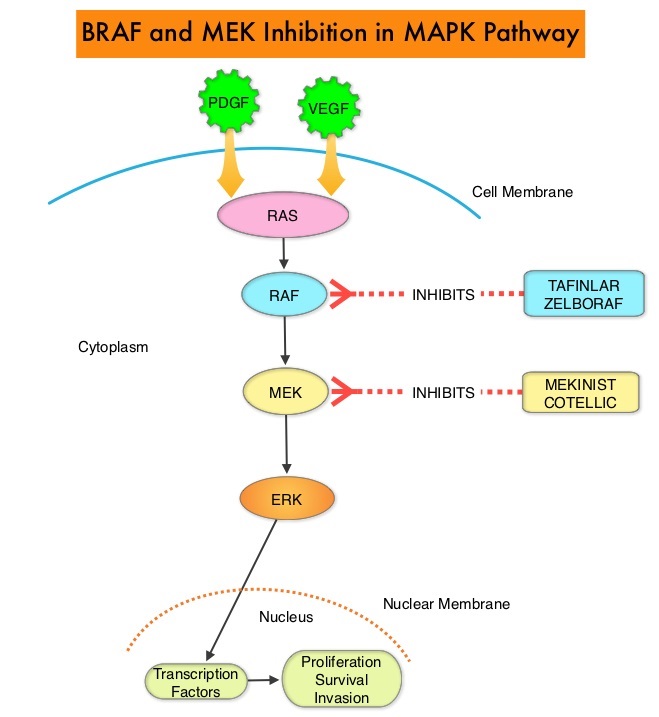
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been detected in 6-8% of all malignancies. The most common BRAF mutation in Melanoma is at the V600E/K site and is detected in approximately 50% of Melanomas, and result in constitutive activation of the MAPK pathway.
TAFINLAR® (Dabrafenib), is a selective oral BRAF inhibitor and MEKINIST® (Trametinib) is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway. It has been well established that patients who have unresectable or metastatic Melanoma with a BRAFV600E or V600K mutation have prolonged PFS and OS when treated with a combination of BRAF and MEK inhibitors. However, long-term 4 and 5-year clinical outcomes in these patient’s have not been reported.
Two randomized Phase III trials helped address this issue. COMBI-d involved 423 patients randomized to TAFINLAR® plus MEKINIST® (N=211) or to TAFINLAR® plus placebo (N=212). In COMBO-v, 704 patients were randomized to TAFINLAR® plus MEKINIST® (N=352) or to single-agent ZELBORAF® (Vemurafenib; N=352). In a previously published pooled analysis of patients treated in the COMBI-d and COMBI-v trials, 3-year PFS and OS were 23% and 44% respectively. Further, there was a significant association between several baseline factors such as performance status, age, sex, number of organ sites with metastasis, serum LDH level and both PFS as well as OS.
In this review, the researchers analyzed pooled long term survival data from two randomized Phase III COMBI-d and COMBI-v trials, which involved previously untreated, unresectable or metastatic Melanoma patients, with BRAFV600E or V600K mutation, who had received BRAF inhibitor TAFINLAR® 150 mg orally twice daily along with a MEK inhibitor MEKINIST® 2 mg orally once daily. These two trials evaluated the efficacy and safety of TAFINLAR® plus MEKINIST®, as compared with BRAF inhibitor monotherapy. The long term, 5-year survival data from these two trials was reported, along with clinical characteristics of the patients who derived long-term benefit from this treatment. The Primary end points in the COMBI-d and COMBI-v trials were PFS and OS, respectively. The median patient age in these trials was 55 years, 3% of patients had nonmetastatic disease and two-thirds had M1c metastatic disease.
A total of 563 patients (211 in the COMBI-d trial and 352 in the COMBI-v trial) were randomly assigned to receive TAFINLAR® plus MEKINIST®. The PFS rates were 21% at 4 years and 19% at 5 years. The OS rates were 37% at 4 years and 34% at 5 years. The 5-year OS rate was 71% among patients who had a Complete Response and 55% among those who had a normal Lactate Dehydrogenase level plus fewer than three metastatic organ sites at baseline.
It was concluded that first-line treatment with TAFINLAR® plus MEKINIST® led to long-term benefit in approximately one third of the patients who had unresectable or metastatic Melanoma with a BRAF V600E or V600K mutation. The authors added that this is the largest data set and longest follow-up in this patient population treated with BRAF and MEK inhibitors. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. Robert C, Grob JJ, Stroyakovskiy D, et al. June 4, 2019. DOI: 10.1056/NEJMoa1904059

