SUMMARY: The FDA on April 17, 2020 granted accelerated approval to PEMAZYRE® (Pemigatinib), for the treatment of adults with previously treated, unresectable locally advanced or metastatic Cholangiocarcinoma with a Fibroblast Growth Factor Receptor 2 (FGFR2) fusion or other rearrangement, as detected by an FDA-approved test. The FDA also approved the FoundationOne® CDX (Foundation Medicine, Inc.), as a companion diagnostic for patient selection.
Bile Duct cancer (Cholangiocarcinoma), comprise about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection, whereas a majority of patients at diagnosis have advanced disease. The 5-year survival is less than 10%, with limited progress made over the past two decades. There is therefore an unmet need for new effective therapies.
FGFRs (Fibroblast Growth Factor Receptors) play an important role in tumor cell proliferation and survival, migration and angiogenesis. Activating fusions, rearrangements, translocations and gene amplifications in FGFRs result in dysregulation of FGFR signaling, and may contribute to the pathogenesis of various cancers, including Cholangiocarcinoma. FGFR2 fusions or rearrangements occur almost exclusively in intrahepatic Cholangiocarcinoma, where they are observed in 10-16% of patients. PEMAZYRE® is a potent, selective, oral kinase inhibitor of FGFR isoforms 1, 2 and 3, which in preclinical studies has demonstrated selective pharmacologic activity against cancer cells with FGFR alterations.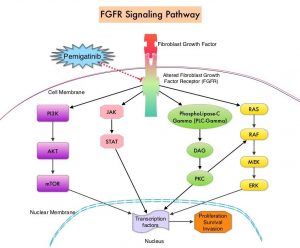
The FIGHT-202 ((FIbroblast Growth factor receptor in oncology and Hematology Trials) is a Phase II, multi-center, open-label, single-arm study which evaluated the safety and efficacy of PEMAZYRE® (Pemigatinib) in adult patients with previously treated, locally advanced or metastatic Cholangiocarcinoma with documented FGFR2 fusion or rearrangement. Patients were enrolled into one of three cohorts: Cohort A with FGFR2 fusions or rearrangements (N=107), Cohort B with other FGF/FGFR genetic alterations (N=20) or Cohort C with no FGF/FGFR genetic alterations (N=18). All patients received PEMAZYRE® 13.5 mg orally once daily, two weeks on and one week off, on a 21-day cycle, until radiological disease progression or unacceptable toxicity. The median patient age of the entire enrolled patient group was 59 years and patients were scanned every eight weeks to assess response to PEMAZYRE®. The Primary endpoint of FIGHT-202 was Objective Response Rate (ORR) in Cohort A, assessed by Independent Review per RECIST criteria. Secondary endpoints included ORR in Cohorts B, A plus B, and C, Duration of Response (DOR), Disease Control Rate (DCR), Progression Free Survival (PFS), Overall Survival (OS), and safety.
It was noted that PEMAZYRE® monotherapy resulted in an Objective Response Rate of 36%, with Complete Response Rate of 2.8% and a Partial Response Rate of 33%. Among those who had a response, 63% had a response lasting 6 months or longer and 18% had a response lasting 12 months or longer. The median Duration of Response was 7.5 months and the Disease Control Rate (DCR) was 82%. The median PFS and median OS were 6.9 months and 21.1 months, and the OS data was not mature at the time of data cutoff. In Cohorts B and C, none of the patients achieved a response. The most common Adverse Events were hyperphosphatemia, alopecia, diarrhea, fatigue, nail toxicities and dysgeusia. Hyperphosphatemia was managed with diet modifications, phosphate binders, diuretics or dose modifications. Fewer patients discontinued therapy in Cohort A compared to Cohort B and C.
It was concluded that based on Overall Response Rate and Duration of Response, PEMAZYRE® is the first and only FDA-approved treatment for previously treated patients with Cholangiocarcinoma, harboring FGFR2 gene rearrangements or fusions.
Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Lancet Oncol. 2020 Mar 20. pii: S1470-2045(20)30109-1. doi: 10.1016/S1470-2045(20)30109-1. [Epub ahead of print]

