The FDA on October 31, 2023, approved KEYTRUDA® to be used with Gemcitabine and Cisplatin for locally advanced unresectable or metastatic Biliary Tract Cancer (BTC). KEYTRUDA® is a product of Merck & Co., Inc.
Tag: Bile Duct Cancer
FDA Approves Pembrolizumab with Chemotherapy for Biliary Tract Cancer
SUMMARY: The FDA on October 31, 2023, approved Pembrolizumab (KEYTRUDA®) to be used with Gemcitabine and Cisplatin for locally advanced unresectable or metastatic Biliary Tract Cancer (BTC). Bile Tract cancer (Cholangiocarcinoma) is a rare and highly aggressive heterogenous cancer, and is the second most common type of primary liver cancer after Hepatocellular carcinoma. It comprises about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. It is estimated that approximately 211,000 patients are diagnosed with Biliary Tract cancer and 174,000 patients will die of the disease each year globally. Biliary Tract cancer is most frequently diagnosed in patients between 50 to 70 years old, and 75% of patients are diagnosed at an advanced stage. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. Patients diagnosed with Biliary Tract cancer have a very poor prognosis, and the 5-year survival among those with advanced stage disease is less than 10%, with limited progress made over the past two decades. There is therefore an urgent unmet need for new effective therapies.
Patients with advanced Biliary Tract cancers often receive chemotherapy in the first and second line settings, with limited benefit. Gemcitabine and Cisplatin combination is currently the first line standard-of-care treatment. With the recognition of immunogenic features displayed by Biliary Tract cancers, the role of immune checkpoint inhibitors for improving disease control and prolonging survival has been increasingly explored.
Pembrolizumab (KEYTRUDA®) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. By doing so, it unleashes the tumor-specific effector T cells, and is thereby able to undo PD-1 pathway-mediated inhibition of the immune response.
KEYNOTE-966 is a multinational, randomized, double-blind, Phase III trial, conducted to determine whether adding the immune checkpoint inhibitor Pembrolizumab to first line standard chemotherapy, would impact survival outcomes in patients with metastatic or unresectable Biliary Tract cancers. In this study, 1069 patients (N=1069) with advanced and/or unresectable Biliary Tract cancers were randomly assigned to receive Pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles (N=533) or placebo (N=536). Both treatment groups received Gemcitabine 1000 mg/m2 IV on days 1 and 8 every 3 weeks without preset maximum number of cycles, and Cisplatin 25 mg/m2 IV on days 1 and 8 every 3 weeks for up to 8 cycles. The median age was 63.5 years, majority of patients had metastatic disease (88%) and more than half had intrahepatic disease. The Primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR), Duration of Response and Safety. The median follow up was 25.6 months.
The median OS was 12.7 months in the Pembrolizumab group and 10.9 months in the placebo group (HR=0.83; P=0.0034). This represented a 17% reduction in the risk of death in the Pembrolizumab group compared to the placebo group. The 12-month OS rate was 52% with the Pembrolizumab regimen versus 44% for chemotherapy alone and the 24-month OS rates were 24.9% versus 18.1%, respectively. The OS results were generally consistent across subgroups.
There was no significant difference in PFS between the treatment groups but there was a trend toward improved PFS with Pembrolizumab. The median PFS was 6.5 months in the Pembrolizumab arm and 5.6 months in the placebo group (HR=0.87; P=0.23). The estimated 12-month PFS was 25% and 20% respectively.The Objective Response Rates were similar between the two treatment groups – 28.7% in the Pembrolizumab group and 28.5% in the placebo arm.The safety profile of Pembrolizumab was consistent with that observed in previously reported studies and Grade 3-4 adverse events were similar between treatment groups.
The authors concluded that KEYNOTE-966 is the largest randomized Phase III trial in advanced Biliary Tract cancers to date, with more patients enrolled from non-Asian countries. First line treatment with Pembrolizumab plus chemotherapy significantly improved Overall Survival, when compared with chemotherapy alone. The researchers added that one of the limitations of this study is that patients with intrahepatic bile duct cancers were overrepresented in the study population compared with the incidence of the disease in the general population, resulting in smaller sample sizes of patients with extrahepatic and gall bladder sites of origin.
Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Kelley RK, Ueno M, Yoo C, et al. The Lancet. 2023;401:1853-1865.
Pembrolizumab Plus Chemotherapy Improves Overall Survival in Advanced Biliary Tract Cancer
SUMMARY: Bile Tract cancer (Cholangiocarcinoma) is a rare and highly aggressive heterogenous cancer, and is the second most common type of primary liver cancer after Hepatocellular carcinoma. It comprises about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. It is estimated that approximately 211,000 patients are diagnosed with Biliary Tract cancer and 174,000 patients will die of the disease each year globally. Biliary Tract cancer is most frequently diagnosed in patients between 50 to 70 years old, and 75% of patients are diagnosed at an advanced stage. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. Patients diagnosed with Biliary Tract cancer have a very poor prognosis, and the 5-year survival among those with advanced stage disease is less than 10%, with limited progress made over the past two decades. There is therefore an urgent unmet need for new effective therapies.
Patients with advanced Biliary Tract cancers often receive chemotherapy in the first and second line settings, with limited benefit. Gemcitabine and Cisplatin combination is currently the first line standard-of-care treatment. With the recognition of immunogenic features displayed by Biliary Tract cancers, the role of immune checkpoint inhibitors for improving disease control and prolonging survival has been increasingly explored.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. By doing so, it unleashes the tumor-specific effector T cells, and is thereby able to undo PD-1 pathway-mediated inhibition of the immune response.
KEYNOTE-966 is a multinational, randomized, double-blind, Phase III trial, conducted to determine whether adding the immune checkpoint inhibitor Pembrolizumab to first line standard chemotherapy, would impact survival outcomes in patients with metastatic or unresectable Biliary Tract cancers. In this study, 1069 patients (N=1069) with advanced and/or unresectable Biliary Tract cancers were randomly assigned to receive Pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles (N=533) or placebo (N=536). Both treatment groups received Gemcitabine 1000 mg/m2 IV on days 1 and 8 every 3 weeks without preset maximum number of cycles, and Cisplatin 25 mg/m2 IV on days 1 and 8 every 3 weeks for up to 8 cycles. The median age was 63.5 years, majority of patients had metastatic disease (88%) and more than half had intrahepatic disease. The Primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR), Duration of Response and Safety. The median follow up was 25.6 months.
The median OS was 12.7 months in the Pembrolizumab group and 10.9 months in the placebo group (HR=0.83; P=0.0034). This represented a 17% reduction in the risk of death in the Pembrolizumab group compared to the placebo group. The 12-month OS rate was 52% with the Pembrolizumab regimen versus 44% for chemotherapy alone and the 24-month OS rates were 24.9% versus 18.1%, respectively. The OS results were generally consistent across subgroups.
There was no significant difference in PFS between the treatment groups but there was a trend toward improved PFS with Pembrolizumab. The median PFS was 6.5 months in the Pembrolizumab arm and 5.6 months in the placebo group (HR=0.87; P=0.23). The estimated 12-month PFS was 25% and 20% respectively.The Objective Response Rates were similar between the two treatment groups – 28.7% in the Pembrolizumab group and 28.5% in the placebo arm.The safety profile of Pembrolizumab was consistent with that observed in previously reported studies and Grade 3-4 adverse events were similar between treatment groups.
The authors concluded that KEYNOTE-966 is the largest randomized Phase III trial in advanced Biliary Tract cancers to date, with more patients enrolled from non-Asian countries. First line treatment with Pembrolizumab plus chemotherapy significantly improved Overall Survival, when compared with chemotherapy alone. The researchers added that one of the limitations of this study is that patients with intrahepatic bile duct cancers were overrepresented in the study population compared with the incidence of the disease in the general population, resulting in smaller sample sizes of patients with extrahepatic and gall bladder sites of origin.
Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Kelley RK, Ueno M, Yoo C, et al. Lancet. Published online April 16, 2023. https://doi.org/10.1016/S0140-6736(23)00727-4.
LYTGOBI® (Futibatinib)
The FDA on September 30, 2022, granted accelerated approval to LYTGOBI® (Futibatinib) for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic Cholangiocarcinoma harboring Fibroblast Growth Factor Receptor 2 (FGFR2) gene fusions or other rearrangements. LYTGOBI® is a product of Taiho Oncology, Inc.
IMFINZI® (Durvalumab)
The FDA on September 2, 2022, approved IMFINZI® (Durvalumab) in combination with Gemcitabine and Cisplatin for adult patients with locally advanced or metastatic Biliary Tract Cancer (BTC). IMFINZI® is a product of AstraZeneca UK Limited.
FDA Grants Accelerated Approval to LYTGOBI® for Cholangiocarcinoma
SUMMARY: The FDA on September 30, 2022, granted accelerated approval to LYTGOBI® (Futibatinib) for adult patients with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma harboring Fibroblast Growth Factor Receptor 2 (FGFR2) gene fusions or other rearrangements. Bile Tract cancer (Cholangiocarcinoma) is a rare, heterogenous cancer, and comprises about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. The 5-year survival among those with advanced stage disease is less than 10%, with limited progress made over the past two decades.
Approximately 75% of patients are diagnosed with late-stage disease, and are often treated with Gemcitabine plus Cisplatin, based on the findings of the ABC-02 study. Second line treatment options include FOLFOX regimen, which is associated with a Response Rate of about 5%, median Progression Free Survival (PFS) of about 4 months, and median Overall Survival (OS) of about 6 months. There is therefore an unmet need for new effective therapies.
FGFRs (Fibroblast Growth Factor Receptors) play an important role in tumor cell proliferation and survival, migration, and angiogenesis. Activating fusions, rearrangements, translocations, and gene amplifications in FGFRs result in dysregulation of FGFR signaling, and may contribute to the pathogenesis of various cancers, including Cholangiocarcinoma. FGFR2 fusions or rearrangements occur almost exclusively in intrahepatic Cholangiocarcinoma, where they are observed in 10-20% of patients, and have been identified as oncogenic drivers. Futibatinib is a highly selective, irreversible FGFR1-4 inhibitor, and demonstrated tolerability and preliminary evidence of clinical efficacy in patients with intrahepatic cholangiocarcinoma.
The present FDA approval was based on the results from the pivotal FOENIX-CCA2 trial (NCT02052778), which is a global, multicenter, open-label, single-arm study that enrolled 103 patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma, harboring a FGFR2 gene fusion or other rearrangement. The presence of FGFR2 fusions or other rearrangements was determined using Next Generation Sequencing testing. Patients received Futibatinib 20 mg orally once daily until disease progression or unacceptable toxicity. The median age was 58 years, 53% had an ECOG Performance Status of 1, all patients had prior anticancer therapy, with 27% receiving prior radiotherapy. FGFR2 fusions were observed in 78% of patients and 22% had a rearrangement. The median time from prior anticancer therapy to the first Futibatinib dose was 1.5 months. The Primary endpoint was Objective Response Rate (ORR) by Independent Central Review. Secondary endpoints were Duration of Response (DOR), Disease Control Rate (DCR), Progression Free Survival (PFS), Overall Survival (OS), Safety, and Patient-Reported Outcomes. At the primary analysis of this trial, an Objective Response Rate of 41.7% was observed, with a median Duration of Response of 9.7 months. The researchers herein reported updated efficacy, including mature Overall Survival, and safety data from the final analysis, with an additional 8 months of follow up.
At a median follow up of 25 months, the median number of treatment cycles was 13.0 and the median treatment duration was 9.1 months. The confirmed Objective Response Rate was 41.7%, like what was noted at the time of primary analysis, and this benefit was consistent across patient subgroups. The Disease Control Rate of 82.5% and was similar as well. The median Duration of Response was 9.5 months, and 74% of responses lasted 6 months or more. The median PFS was 8.9 months, with a 12-month PFS rate of 35%. The median Overall Survival was 20 months, with a 12-month Overall Survival rate of 73%. The most common treatment-related adverse events included hyperphosphatemia (85%), alopecia (33%), dry mouth (30%), diarrhea (28%), dry skin (27%), and fatigue (25%). Approximately 4% of patients discontinued treatment due to adverse events.
The authors concluded that the final analysis of FOENIX-CCA2 study confirmed the results of the primary analysis and reinforced the durable efficacy and continued tolerability of Futibatinib in previously treated patients with advanced/metastatic intrahepatic cholangiocarcinoma harboring FGFR2 fusion/rearrangements. They added that the mature Overall Survival far exceeded historical data in this patient population.
Updated results of the FOENIX-CCA2 trial: Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/rearrangements. Goyal L, Meric-Bernstam F, Hollebecque A, et al. J ClinOncol. 2022;40(suppl 16):4009. doi:10.1200/JCO.2022.40.16_suppl.4009
FDA Approves IMFINZI® in Combination with Chemotherapy for Advanced Biliary Tract Cancer
SUMMARY: The FDA on September 2, 2022, approved IMFINZI® (Durvalumab) in combination with Gemcitabine and Cisplatin for adult patients with locally advanced or metastatic Biliary Tract cancer. Bile Tract cancer (Cholangiocarcinoma) is a rare, heterogenous cancer, and comprises about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. The 5-year survival among those with advanced stage disease is less than 10%, with limited progress made over the past two decades. There is therefore an unmet need for new effective therapies.
Patients with advanced Biliary Tract cancers often receive chemotherapy in the first and second line settings, with limited benefit. Gemcitabine and Cisplatin combination is currently the first line standard-of-care treatment. With the recognition of immunogenic features displayed by Biliary Tract cancers, the role of immune checkpoint inhibitors for improving disease control and prolonging survival, has been increasingly explored.
IMFINZI® (Durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins, countering the tumor’s immune-evading tactics and unleashes the T cells. IMFINZI® in combination with Gemcitabine and Cisplatin showed encouraging antitumor activity in a Phase II study, among patients with advanced Biliary Tract cancers.
TOPAZ-1 is a double-blind, multicenter, global, Phase III trial conducted to evaluate the efficacy of first line immunotherapy given along with Gemcitabine and Cisplatin in patients with advanced metastatic Biliary Tract cancer. In this study, a total of 685 previously untreated patients with unresectable, locally advanced, recurrent or metastatic Biliary Tract cancer were randomized 1:1 to receive IMFINZI® (Durvalumab) 1500 mg IV every 3 weeks (N=341) or placebo (N=344), along with Gemcitabine 1000 mg/m2 IV and Cisplatin 25 mg/m2 IV given on Days 1 and 8, every 3 weeks for up to 8 cycles, followed by IMFINZI® 1500 mg IV every 4 weeks or placebo, until disease progression or unacceptable toxicity. Patients with recurrent disease more than 6 months following curative surgery or adjuvant therapy were also included. The median patient age was 64 years and approximately 50% of patients had an ECOG Performance Status of 0. Randomization was stratified by disease status (initially unresectable, recurrent) and primary tumor location (intrahepatic cholangiocarcinoma versus extrahepatic cholangiocarcinoma versus gallbladder cancer). Approximately 56% had intrahepatic cholangiocarcinoma, followed by gallbladder cancer (25%) and extrahepatic cholangiocarcinoma (19%). Tumor assessments were conducted every 6 weeks for the first 24 weeks, and then every 8 weeks until confirmed objective disease progression. The Primary endpoint was Overall Survival (OS) and Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR), and Safety.
IMFINZI® plus Gemcitabine and Cisplatin significantly improved Overall Survival compared with placebo plus chemotherapy, with a 20% reduction in the risk of death. The median OS was 12.8 months and 11.5 months in the IMFINZI® and placebo groups, respectively (HR=0.80; P=0.021). The median PFS was 7.2 months and 5.7 months in the IMFINZI® and placebo arms, respectively (HR=0.75; P=0.001). The Objective Response Rate was 26.7% in the IMFINZI® plus chemotherapy group and 18.7% in the placebo plus chemotherapy group. Grade 3 or 4 treatment-related adverse events were almost similar in both treatment groups (62.7% versus 64.9%), and treatment discontinuation due to adverse events was 8.9% in the IMFINZI® plus chemotherapy group and 11.4% in the placebo plus chemotherapy group.
It was concluded that in patients with advanced Biliary Tract cancers, IMFINZI® in combination with Gemcitabine and Cisplatin significantly improved Overall Survival and Progression Free Survival with manageable safety, when compared to chemotherapy alone, and should therefore be considered first line standard-of- care for this patient group.
A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (gemcis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. Oh D-Y, He AR, Qin S, et al. J Clin Oncol. 2022;40(suppl 4):378. DOI:10.1200/JCO.2022.40.4_suppl.378.
IMFINZI® in Combination with Chemotherapy Improves Overall Survival in Advanced Biliary Tract Cancer
SUMMARY: Bile Tract cancer (Cholangiocarcinoma) is a rare, heterogenous cancer, and comprises about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. The 5-year survival among those with advanced stage disease is less than 10%, with limited progress made over the past two decades. There is therefore an unmet need for new effective therapies.
Patients with advanced bile tract cancers often receive chemotherapy in the first and second line settings, with limited benefit. Gemcitabine and Cisplatin combination is currently the first line standard-of-care treatment. With the recognition of immunogenic features displayed by bile tract cancers, the role of immune checkpoint inhibitors for improving disease control and prolonging survival, has been increasingly explored.
IMFINZI® (Durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins, countering the tumor’s immune-evading tactics and unleashes the T cells. IMFINZI® in combination with Gemcitabine and Cisplatin showed encouraging antitumor activity in a Phase II study, among patients with advanced bile tract cancers.
TOPAZ-1 is a double-blind, multicenter, global, Phase III trial conducted to evaluate the efficacy of first line immunotherapy given along with Gemcitabine and Cisplatin in patients with advanced metastatic biliary tract cancer. In this study, a total of 685 previously untreated patients with unresectable, locally advanced, recurrent or metastatic biliary tract cancer were randomized 1:1 to receive IMFINZI® (Durvalumab) 1500 mg IV every 3 weeks (N=341) or placebo (N=344), along with Gemcitabine 1000 mg/m2 IV and Cisplatin 25 mg/m2 IV given on Days 1 and 8, every 3 weeks for up to 8 cycles, followed by IMFINZI® 1500 mg IV every 4 weeks or placebo, until disease progression or unacceptable toxicity. Patients with recurrent disease more than 6 months following curative surgery or adjuvant therapy were also included. The median patient age was 64 years and approximately 50% of patients had an ECOG Performance Status of 0. Randomization was stratified by disease status (initially unresectable, recurrent) and primary tumor location (intrahepatic cholangiocarcinoma versus extrahepatic cholangiocarcinoma versus gallbladder cancer). Approximately 56% had intrahepatic cholangiocarcinoma, followed by gallbladder cancer (25%) and extrahepatic cholangiocarcinoma (19%). The Primary endpoint was Overall Survival (OS) and Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR), and Safety.
The Primary endpoint was met at the first interim analysis and treatment with IMFINZI® plus Gemcitabine and Cisplatin significantly improved Overall Survival compared with placebo plus chemotherapy, with a 20% reduction in the risk of death (HR=0.80; P=0.021). Progression Free Survival was also greatly improved with chemoimmunotherapy vs chemotherapy alone (HR=0.75; P=0.001). The Objective Response Rate was was 26.7% in the IMFINZI® plus chemotherapy group and 18.7% in the placebo plus chemotherapy group. Grade 3 or 4 treatment-related adverse events were almost similar in both treatment groups (62.7% versus 64.9%), and treatment discontinuation due to adverse events was 8.9% in the IMFINZI® plus chemotherapy group and 11.4% in the placebo plus chemotherapy group.
It was concluded that in patients with advanced biliary tract cancers, IMFINZI® in combination with Gemcitabine and Cisplatin significantly improved Overall Survival and Progression Free Survival with manageable safety, when compared to chemotherapy alone, and should therefore be considered first line standard-of- care for this patient group.
A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (gemcis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. Oh D-Y, He AR, Qin S, et al. J Clin Oncol. 2022;40(suppl 4):378. DOI:10.1200/JCO.2022.40.4_suppl.378.
FDA Approves TIBSOVO® for IDH1 Mutated Advanced Cholangiocarcinoma
SUMMARY: The FDA on August 25, 2021, approved TIBSOVO® (Ivosidenib) for adult patients with previously treated, locally advanced or metastatic Cholangiocarcinoma, with an Isocitrate DeHydrogenase-1 (IDH1) mutation, as detected by an FDA-approved test. The FDA also approved the Oncomine Dx Target Test (Life Technologies Corporation) as a companion diagnostic device, to aid in selecting patients with Cholangiocarcinoma for treatment with TIBSOVO®.
Bile Duct cancer (Cholangiocarcinoma), comprise about 30% of all primary liver tumors and includes both intrahepatic and extrahepatic bile duct cancers. Klatskin tumor is a type of Cholangiocarcinoma that begins in the hilum, at the junction of the left and right bile ducts. It is the most common type of Cholangiocarcinoma, accounting for more than half of all cases. About 8,000 people in the US are diagnosed with Cholangiocarcinoma each year and approximately 20% of the cases are suitable for surgical resection. The 5-year survival is less than 10%, with limited progress made over the past two decades. There is therefore an unmet need for new effective therapies.
Isocitrate DeHydrogenase (IDH) is a metabolic enzyme that helps generate energy from glucose and other metabolites, by catalyzing the conversion of Isocitrate to Alpha-Ketoglutarate. Alpha-ketoglutarate is required to properly regulate DNA and histone methylation, which in turn is important for gene expression and cellular differentiation. IDH mutations lead to aberrant DNA methylation and altered gene expression thereby preventing cellular differentiation, with resulting immature undifferentiated cells. IDH mutations can thus promote leukemogenesis in Acute Myeloid Leukemia (AML) and tumorigenesis in solid tumors and can result in inferior outcomes. There are three isoforms of IDH. IDH1 is mainly found in the cytoplasm, as well as in peroxisomes, whereas IDH2 and IDH3 are found in the mitochondria, and are a part of the Krebs cycle. Approximately 20% of patients with AML, 70% of patients with Low-grade Glioma and secondary Glioblastoma, 50% of patients with Chondrosarcoma, 20% of patients with Intrahepatic Cholangiocarcinoma, 30% of patients with Angioimmunoblastic T-cell lymphoma and 8% of patients with Myelodysplastic syndromes/Myeloproliferative neoplasms, are associated with IDH mutations.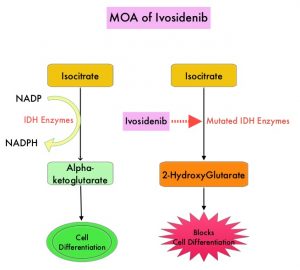
TIBSOVO® (Ivosidenib) is an oral, targeted, small-molecule inhibitor of mutant IDH1. A previously published Phase I study demonstrated the safety and activity of TIBSOVO® in patients with IDH1 mutated advanced Cholangiocarcinoma. ClarIDHy is an international, randomized, double-blind, Phase III study, in which 187 previously treated patients with advanced Cholangiocarcinoma with an IDH1 mutation were randomly assigned 2:1 to receive TIBSOVO® 500 mg orally once daily (N=126) or matched placebo (N=61). All patients had advanced unresectable Cholangiocarcinoma. The median age was 62 years, 91% had intrahepatic Cholangiocarcinoma, 93% of patients had metastatic disease and 47% had received two prior therapies. The patient’s disease must have progressed following at least one, but not more than two prior regimens, including at least one Gemcitabine or 5-FU containing regimen. The Primary endpoint was Progression Free Survival (PFS) and Secondary endpoints included Safety, Objective Response Rate (ORR) and Overall Survival (OS). Crossover from placebo to TIBSOVO® was permitted upon radiographic disease progression.
This study met its Primary endpoint and the median PFS was 2.7 months for patients treated with TIBSOVO® compared to 1.4 months with placebo (HR=0.37; P<0.0001). More importantly, the median PFS at 6 and 12 months were 32% and 22% in the TIBSOVO® group, whereas no patients randomized to the placebo group were progression-free for 6 or more months, at the time of data cutoff.
The authors also reported the results of final analysis which showed an improvement in the secondary endpoint of OS, favoring patients randomized to TIBSOVO® compared to those randomized to placebo. However, statistical significance was not reached. The median OS for patients in the TIBSOVO® arm was 10.3 months compared to 7.5 months for patients in the placebo arm (HR=0.79; 1-sided P=0.093). A high proportion of patients in the placebo arm (70.5%) crossed over to TIBSOVO®. After adjusting for crossover from placebo to TIBSOVO®, the median OS for patients in the placebo arm was 5.1 months (HR=0.49; 1-sided P<0.0001).
The 6-month survival rate for patients in the TIBSOVO® arm was 69% compared to 57% of patients in the placebo arm, not adjusted for crossover. The 12-month survival rate for patients in the TIBSOVO® arm was 43% compared to 36% for patients in the placebo arm, not adjusted for crossover. Treatment with TIBSOVO® preserved patients’ physical functioning from baseline, as assessed by the EORTC QLQ-C30 questionnaire, whereas patients in the placebo arm experienced decline from baseline starting cycle 2. The most common Adverse Events of any grade for TIBSOVO® were nausea (38%), diarrhea (33.1%) and fatigue (28.9%). Adverse Events leading to discontinuation were more common with placebo compared with total TIBSOVO® (8.5% versus 6.6%).
It was concluded that treatment with TIBSOVO® in patients with advanced Cholangiocarcinoma with an IDH1 mutation, resulted in significant improvement in Progression Free Survival as well as favorable Overall Survival trend, when compared to Placebo, despite a high rate of crossover. This is the first pivotal study demonstrating the clinical benefit of targeting IDH1 mutation in this patient group. This new oral, non-cytotoxic, targeted treatment option, with a tolerable safety profile, will be a welcome addition to treat this aggressive disease, for which there is an unmet need for new therapies.
Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. Zhu A, Macarulla T, Javle MM, et al. J Clin Oncol 39, 2021 (suppl 3; abstr 266)
TIBSOVO® (Ivosidenib)
The FDA on August 25, 2021 approved TIBSOVO® (Ivosidenib) for adult patients with previously treated, locally advanced or metastatic Cholangiocarcinoma with an Isocitrate DeHydrogenase-1 (IDH1) mutation, as detected by an FDA-approved test. TIBSOVO® is a product of Servier Pharmaceuticals, LLC.
