SUMMARY: The FDA on February 25, 2020, approved NERLYNX® (Neratinib) in combination with XELODA® (Capecitabine) for adult patients with advanced or metastatic HER2-positive breast cancer, who have received two or more prior anti-HER2 based regimens in the metastatic setting. Breast cancer is the most common cancer among women in the US and about 1 in 8 women (13%) will develop invasive breast cancer during their lifetime. Approximately 276,480 new cases of invasive female breast cancer will be diagnosed in 2020 and about 42,170 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. Patients with HER2-positive metastatic breast cancer are often treated with anti-HER2 targeted therapy along with chemotherapy, irrespective of hormone receptor status, and this has resulted in significantly improved treatment outcomes.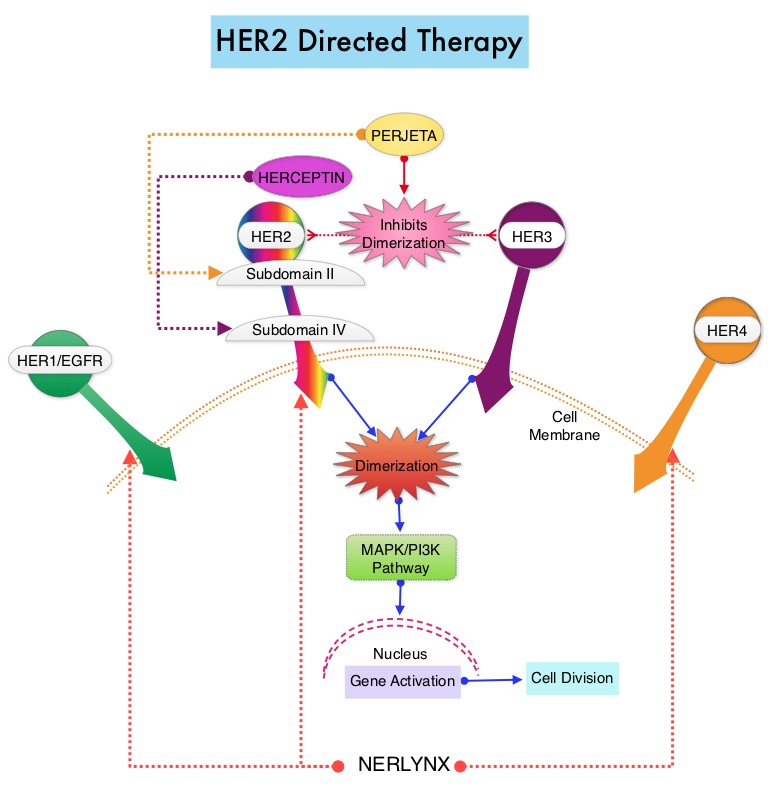
NERLYNX® (Neratinib) is a potent, irreversible, oral Tyrosine Kinase Inhibitor, of HER1, HER2 and HER4 (pan-HER inhibitor). NERLYNX® interacts with the catalytic domain of HER1, HER2, and HER4 and blocks their downstream signaling pathways, resulting in decreased cell proliferation and increased cell death. Clinical data has suggested that NERLYNX® has significant activity in suppressing HER-mediated tumor growth and is able to overcome tumor escape mechanisms experienced with current HER2-targeted and chemotherapeutic agents. It has been well known that hormone receptor positive breast cancer patients, who are also HER2-positive, have relative resistance to hormone therapy. Preclinical models had suggested that the addition of NERLYNX® could improve responses in ER positive, HER2-positive breast cancer patients. Further, NERLYNX® has clinical activity in patients with HER2-positive metastatic breast cancer. NERLYNX® is the first TKI approved by the FDA, shown to reduce the risk for disease recurrence, in patients with early stage HER2-positive breast cancer. NERLYNX® when given for 12 months after chemotherapy and HERCEPTIN®-based adjuvant therapy, to women with HER2-positive breast cancer, significantly reduced the proportion of clinically relevant breast cancer relapses that might lead to death, such as distant and locoregional recurrences outside the preserved breast.
TYKERB® (Lapatinib) is a Tyrosine Kinase Inhibitor of HER2 and EGFR, and in a previously published Phase III study, a combination of TYKERB® plus XELODA® (Capecitabine) was found to be superior to XELODA® alone in women with HER2-positive advanced breast cancer, that has progressed after treatment with regimens that included an Anthracycline, a Taxane, and HERCEPTIN®. (N Engl J Med 2006; 355:2733-2743)
NALA is a multinational, randomized, open-label, Phase III trial of NERLYNX® plus XELODA® in patients with heavily pretreated Stage IV HER2-positive metastatic breast cancer. In this study, 621 patients with metastatic HER2-positive breast cancer who received two or more prior anti-HER2 based regimens in the metastatic setting were randomly assigned in a 1:1 to receive NERLYNX® 240 mg given orally once daily on days 1-21 along with XELODA® 750 mg/m2 given orally twice daily on days 1-14 for each 21-day cycle (N=307) or TYKERB® 1250 mg given orally once daily on days 1-21 along with XELODA® 1000 mg/m2 given orally twice daily on days 1-14 for each 21-day cycle (N=314). Approximately 80% had visceral metastases, and about 30% had received at least three anti-HER2 therapies. Patients in the NERLYNX® group also received antidiarrheal prophylaxis with Loperamide. Patients were treated until disease progression or unacceptable toxicity. The Co-Primary endpoints were Progression Free Survival (PFS) and Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR) and Duration of Response, Clinical Benefit Rate (CBR), time to intervention for symptomatic metastatic Central Nervous System (CNS) disease and Safety.
It was noted that the median PFS was 5.6 months for patients who received NERLYNX® with XELODA® and 5.5 months for those receiving TYKERB® with XELODA® (HR=0.76; P=0.006). This represented a 24% reduction in the risk of disease progression or death for those receiving a combination of NERLYNX® and XELODA®. The PFS rate at 12 months was 29% versus 15% respectively. The median OS was 21 months for patients receiving NERLYNX® and XELODA® compared to 18.7 months for those receiving TYKERB® and XELODA® (HR=0.88; P=0.20). The ORR was numerically higher with NERLYNX® and XELODA® combination in patients with measurable disease (32.8% versus 26.7%), and there was a statistically significant improvement in the Clinical Benefit Rate (45% versus 36%; P=0.03). The median Duration of Response was 8.5 versus 5.6 months respectively (HR=0.50; P=0.0004), favoring the NERLYNX® combination. The time to intervention for symptomatic CNS disease was significantly delayed with NERLYNX® combination versus TYKERB® combination, with an overall cumulative incidence of 22.8% versus 29.2% respectively (P= 0.043). Treatment related toxicities were similar between arms, but there was a higher rate of Grade 3 diarrhea with the NERLYNX® combination (24.4% versus 12.5% respectively). Discontinuation due to treatment related toxicities were lower with NERLYNX® combination versus TYKERB® combination (10.9% versus 14.5%).
It was concluded from this study that a combination of NERLYNX® and XELODA® significantly improved Progression Free Survival, with a trend towards improved Overall Survival, and also resulted in a delayed time to intervention for symptomatic CNS disease, among patients with heavily pretreated advanced HER2-positive breast cancer. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. Saura C, Oliveira M, Feng Y-H, et al. J Clin Oncol. 2019;37 (suppl; abstr 1002).

