SUMMARY: The Centers for Disease Control and Prevention estimates that in the US, there are more than 16,000 cases of Human PapillomaVirus (HPV)-positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) per year and there has been a signiï¬cant increase in incidence during the past several decades. They represent approximately 70% of all OPSCC in the United States and Canada. HPV is a non-enveloped, double-stranded, DNA virus that infects epithelial cells and majority of the HPV subtypes cause epithelial lesions such as warts or papillomas, which are of low malignant potential. However, there is a subset of high-risk HPV that can cause cancer by integrating its DNA into the host genome, with the resulting expression of two important oncogenes E6 and E7 in the host cell. The E6 oncogene binds and degrades tumor suppressor TP53 via ubiquitin-mediated processes thereby preventing the host cell from engaging in cell cycle checkpoints and enduring an apoptotic response. The E7 oncogene binds to and destabilizes tumor suppressor retinoblastoma (pRb) resulting in transcription of genes involved in proliferation and cell cycle progression. One of the main molecular pathways amplified through E7 is the CDKN2A/p16 gene pathway, which results in the overexpression of p16 protein. E7 also induces cellular proliferation by disrupting the activity of Cyclin Dependent Kinase inhibitors p21 and p27. In essence, HPV infection induces failures in cell cycle checkpoints, resulting in genetic instability and over time, progression of premalignant lesions to invasive Squamous Cell Carcinoma. Unlike tobacco induced HNSCC where TP53 and pRb pathways are nullified due to mutation and epigenetic alterations, in HPV-related HNSCC, wild-type TP53 and pRb are functionally inactivated by the viral oncogenes.
Patients with HPV-positive OPSCC tend to be younger males, who are former smokers or nonsmokers, with risk factors for exposure to High Risk HPV infection. The HPV-positive primary Squamous Cell Carcinoma tends to be smaller in size, with early nodal metastases, and HPV status particularly in OroPharyngeal Squamous Cell Carcinoma is an independent prognostic factor for Overall Survival (OS) and Progression Free Survival (PFS). These patients have a better prognosis compared with patients with HPV-negative Head and Neck Squamous Cell Carcinoma (HNSCC), when treated similarly. Further, HPV positive patients demonstrate higher Response Rates to chemoradiation as well as an improved Overall Survival. However, approximately 20% of patients diagnosed with HPV-positive OPSCC experience cancer progression within 5 years.
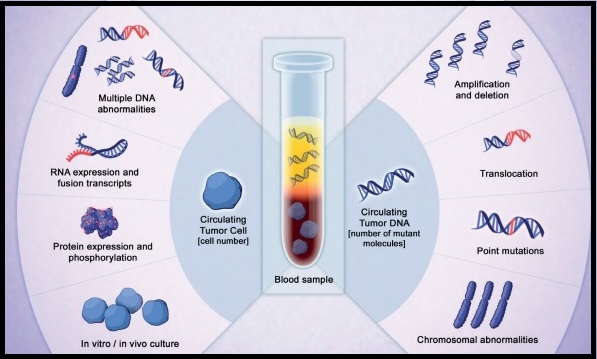
The role of circulating tumor DNA (ctDNA) as a cancer biomarker in the post-operative surveillance of patients with HPV-associated OPSCC has remained unclear. The authors in this study aimed to investigate ctDNA detectability rates by post-op risk category and association with prognosis in this patient population. The researchers prospectively collected and tested serum samples from 29 patients with HPV-associated OPSCC who had not yet undergone treatment, for assay validation. As a control, 7 HPV negative OPSCC patients were included. A cohort of 46 patients with HPV-associated OPSCC who had undergone surgery for the disease had serum samples collected prior to beginning adjuvant therapy. The serum collected from this total group of 82 patients (N=29+7+46) was analyzed in a blinded fashion for E6/E7 HPV ctDNA using ddPCR multiplex assay (HPV 16, 18, 31, 33), and HPV ctDNA detectability was compared statistically across groups. Associations of patient and tumor characteristics with recurrence were assessed and estimates of Progression Free Survival (PFS) and Overall Survival (OS) were made using the Kaplan-Meier (KM) method.
The researchers found that ctDNA was detectable in 27 of 29 patients who had not yet undergone treatment, for a sensitivity rate of 93%, whereas none of the 7 HPV-negative patients had detectable ctDNA, for a specificity rate of 100%. Post-op serum was collected at a median of 25 days after surgery prior to beginning adjuvant therapy and ctDNA was detectable in 43% of patients including 47% with high-risk features (Extra Nodal Extension or R1-microscopic residual tumor). All detected ctDNA was HPV type 16.
At a median follow up of 20 months for the post-op HPV-OPSCC cohort, 24% of these patients had recurrent disease. Among those who recurred, 64% had detectable ctDNA compared with 35% whose disease did not recur (P=0.1). There was a significant association between detectable ctDNA and 24-month Progression Free Survival (45% versus 84%; P=0.04) and Overall Survival (80% versus 100%; P=0.02). Overall, detectable ctDNA, T4 tumors, and more than 4 positive lymph nodes were positively associated with disease recurrence.
It was concluded that detectable HPV circulating tumor DNA is a highly sensitive and specific means of determining HPV-status and was significantly associated with worsened Progression Free Survival and Overall Survival among post-op patients with Human Papilloma Virus (HPV)-associated OroPharyngeal Squamous Cell Carcinoma. HPV circulating tumor DNA as a cancer biomarker may also assist in risk stratification, treatment assessment, and surveillance. Detectable HPV ctDNA in Post-Operative Oropharyngeal Squamous Cell Carcinoma Patients is Associated with Progression. Routman DM, Chera BS, Jethwa KR, et al. International Journal of Radiation Oncology • Biology • Physics 2019;105, 682-683. LBA5