SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000-100,000 deaths. Venous ThromboEmbolism (VTE) is the third leading cause of cardiovascular mortality. Clinicians are often confronted with the dilemma of using bridge therapy for patients with a history of VTE on Warfarin therapy, requiring invasive diagnostic or surgical procedures. Bridge therapy involves the use of rapid onset, short acting anticoagulant preparation such as Low Molecular Weight Heparin during the peri-procedural period (usually 5-10 days), to minimize the risk of subtherapeutic anticoagulation, during peri-procedure Warfarin withdrawal and reinitiation. Even though published studies have largely reviewed the risk of thrombotic events in patients with atrial fibrillation or mechanical heart valves, the risk for bleeding and VTE, associated with bridge therapy in patients receiving Warfarin for the secondary prevention of VTE, has remained unclear. Presently available guidelines that classify peri-procedural risk of recurrent VTE, off anticoagulant therapy, into high risk , medium risk and low risk, is based on indirect, low quality evidence and is not completely reliable. It is well established however, that anticoagulants stopped during the first four weeks of treatment, following diagnosis of VTE, predisposes an individual to increased risk of recurrent VTE.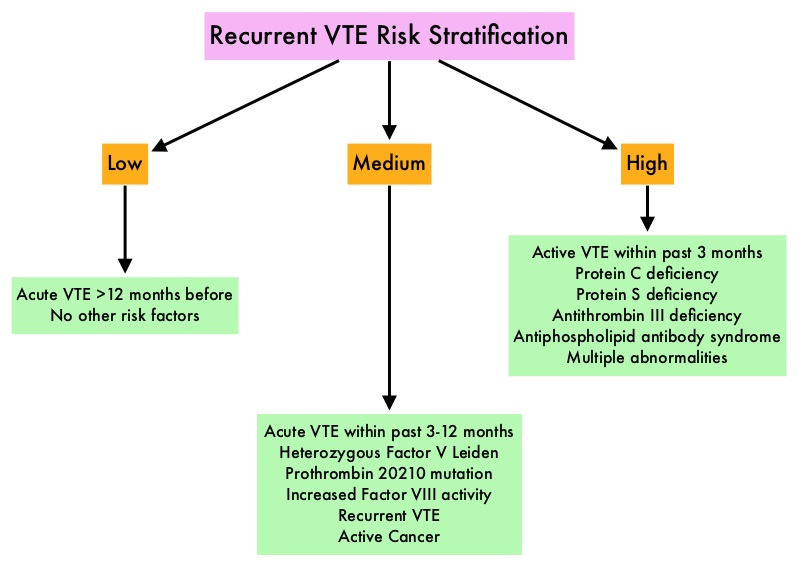
To clarify the risk/benefits of bridge therapy, the authors conducted a retrospective cohort study, to assess the rates of clinically relevant bleeding and recurrent VTE, among patients in whom Warfarin therapy was interrupted for invasive procedures. They then compared the incidence of peri-procedure bleeding and recurrent VTE when a bridging strategy was used or not used. This study included 1178 patients and the most common indication for Warfarin therapy was DVT (56.3%). Majority of the patients (79%) were considered as low risk (acute VTE more than 12 months previously, with no other risk factors). The primary outcome of the study was clinically relevant bleeding resulting in hospitalization, ER visit or complicating the procedure in the first 30 days following the index procedure. Secondary outcomes included recurrent VTE, and all-cause mortality occurring in up to 30 days, following the index procedure.
It was noted that the 30-day rate of clinically relevant bleeding was significantly higher in the bridging group compared with the control group (2.7% versus 0.2%, HR=17.2; P=0.01). Further, there was no significant difference in the rate of recurrent VTE between the bridge and non-bridge therapy groups. No deaths occurred in either treatment groups. The authors concluded that bridge therapy for invasive procedures in patients receiving Warfarin for secondary VTE prevention, is associated with a significant increase in the incidence of bleeding complications, without any decrease in the rate of recurrent VTE. Warfarin can be safely interrupted in this patient group without bridge therapy. Further research is needed to address the value of bridge therapy in the medium and high risk groups. Bleeding, Recurrent Venous Thromboembolism, and Mortality Risks During Warfarin Interruption for Invasive Procedures. Clark NP, Witt DM, Davies LE, et al. JAMA Intern Med 2015;175:1163-1168.

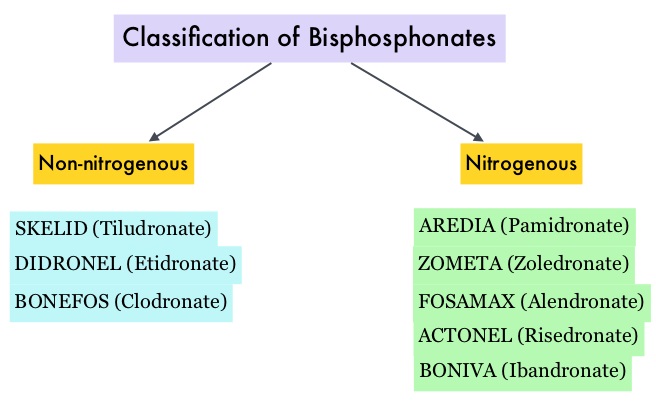 Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.
Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.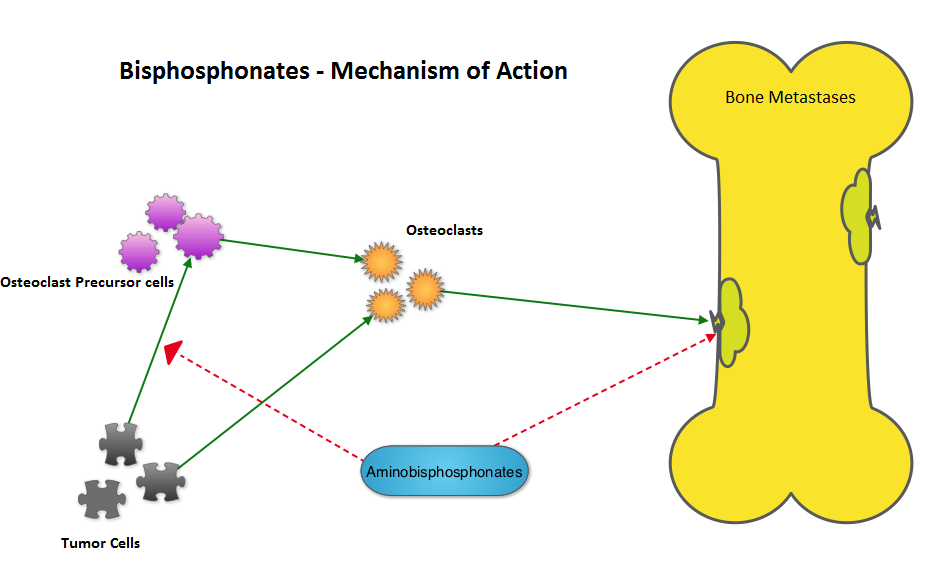 The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.
The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment. The beneficial effects of Vitamin D in malignancies has been attributed to its antiproliferative and antiangiogenic properties, as well as its effects on cell differentiation, promotion of apoptosis and its ability to decreases oxidative DNA damage. Further, macrophages play an important role in the human body’s response to therapy with monoclonal antibodies, an integral part of Follicular Lymphoma therapies and low serum Vitamin D levels may interfere with macrophage function and this may explain poor outcomes in some Follicular Lymphoma patients with low Vitamin D levels
The beneficial effects of Vitamin D in malignancies has been attributed to its antiproliferative and antiangiogenic properties, as well as its effects on cell differentiation, promotion of apoptosis and its ability to decreases oxidative DNA damage. Further, macrophages play an important role in the human body’s response to therapy with monoclonal antibodies, an integral part of Follicular Lymphoma therapies and low serum Vitamin D levels may interfere with macrophage function and this may explain poor outcomes in some Follicular Lymphoma patients with low Vitamin D levels They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). ZYTIGA® inhibits CYP 17A1 enzyme thus decreasing androgen biosynthesis and depletes adrenal and intratumoral androgens. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor (AR), thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. There is presently very little guidance with regards to the sequencing of these two oral agents after progression on TAXOTERE®, in patients with metastatic CRPC. ZYTIGA® was approved initially by the FDA in April 2011, for use in combination with prednisone for the treatment of patients with metastatic CRPC, who had received prior chemotherapy containing TAXOTERE®. Treatment with ZYTIGA® resulted in a 35% reduction in the risk of death and a 36% increase in median Overall Survival (OS) compared with placebo. Subsequently, XTANDI® was approved by the FDA on August 31, 2012 for the treatment of patients with metastatic CRPC who had previously received TAXOTERE®. XTANDI® improved median OS and reduced the risk of death by 37% when compared to placebo. Even though these two anti-androgen therapies improved OS in metastatic CRPC patients previously treated with TAXOTERE®, the proper sequence of administration of these two agents after TAXOTERE® failure, has remained unclear. At least 2 published studies have shown that the use of ZYTIGA® as third line therapy after progression on TAXOTERE® and XTANDI® resulted in inferior outcomes.
They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). ZYTIGA® inhibits CYP 17A1 enzyme thus decreasing androgen biosynthesis and depletes adrenal and intratumoral androgens. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor (AR), thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. There is presently very little guidance with regards to the sequencing of these two oral agents after progression on TAXOTERE®, in patients with metastatic CRPC. ZYTIGA® was approved initially by the FDA in April 2011, for use in combination with prednisone for the treatment of patients with metastatic CRPC, who had received prior chemotherapy containing TAXOTERE®. Treatment with ZYTIGA® resulted in a 35% reduction in the risk of death and a 36% increase in median Overall Survival (OS) compared with placebo. Subsequently, XTANDI® was approved by the FDA on August 31, 2012 for the treatment of patients with metastatic CRPC who had previously received TAXOTERE®. XTANDI® improved median OS and reduced the risk of death by 37% when compared to placebo. Even though these two anti-androgen therapies improved OS in metastatic CRPC patients previously treated with TAXOTERE®, the proper sequence of administration of these two agents after TAXOTERE® failure, has remained unclear. At least 2 published studies have shown that the use of ZYTIGA® as third line therapy after progression on TAXOTERE® and XTANDI® resulted in inferior outcomes.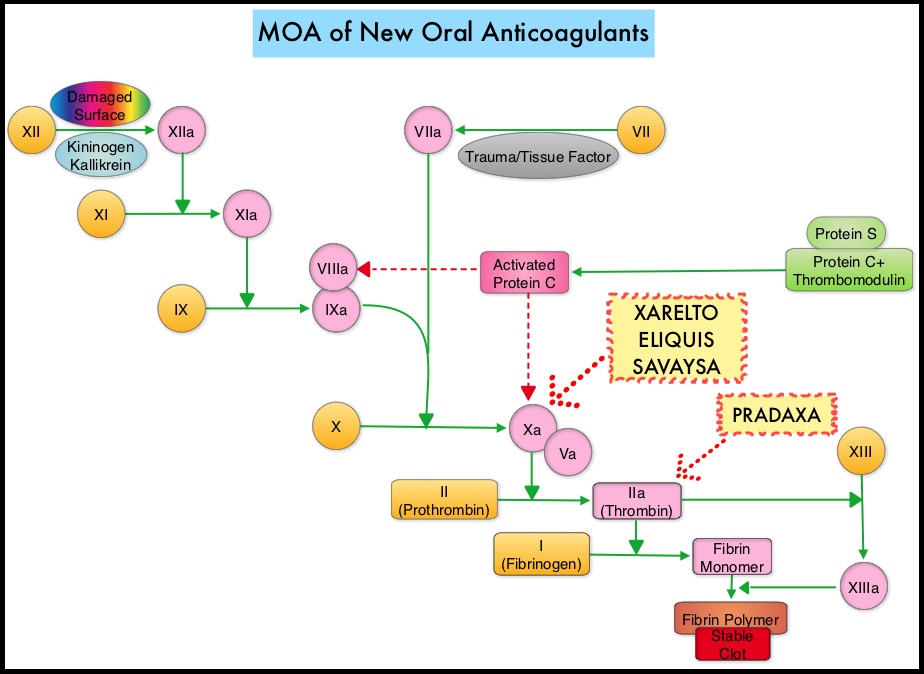 The half life of these agents can however be prolonged in those with renal insufficiency. In several clinical studies, these New Oral Anticoagulants have been shown to reduce the rate of major bleeding by 28% and the rates of intracranial and fatal hemorrhage by 50%, when compared to COUMADIN®. Unlike bleeding caused by COUMADIN®, which can be reversed using Vitamin K or Fresh Frozen Plasma, there are no specific agents presently available, for reversing bleeding caused by the New Oral Anticoagulants or for stopping the anticoagulant effects of these drugs, in patients who need urgent s
The half life of these agents can however be prolonged in those with renal insufficiency. In several clinical studies, these New Oral Anticoagulants have been shown to reduce the rate of major bleeding by 28% and the rates of intracranial and fatal hemorrhage by 50%, when compared to COUMADIN®. Unlike bleeding caused by COUMADIN®, which can be reversed using Vitamin K or Fresh Frozen Plasma, there are no specific agents presently available, for reversing bleeding caused by the New Oral Anticoagulants or for stopping the anticoagulant effects of these drugs, in patients who need urgent s