SUMMARY: The FDA on May 17, 2016, granted accelerated approval to OPDIVO® (Nivolumab) for the treatment of patients with Classical Hodgkin Lymphoma (cHL) that has relapsed or progressed after autologous Hematopoietic Stem Cell Transplantation (HSCT) and post-transplantation ADCETRIS® (Brentuximab vedotin). The American Cancer Society estimates that in the United States for 2016, about 8500 new cases of Hodgkin lymphoma will be diagnosed and over 1100 patients will die of the disease. Hodgkin lymphoma is classified into two main groups-Classical Hodgkin lymphomas and Nodular Lymphocyte Predominant type, by the World Health Organization. The Classical Hodgkin lymphomas include Nodular sclerosing, Mixed cellularity, Lymphocyte rich, Lymphocyte depleted subtypes and accounts for approximately 10% of all malignant lymphomas. Nodular sclerosis Hodgkin lymphoma histology, accounts for approximately 80% of Hodgkin lymphoma cases in older children and adolescents in the United States. Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-L. Classical Hodgkin Lymphoma is an excellent example of how the tumor microenvironment influences cancer cells to proliferate and survive.
The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells, as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block or inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2.
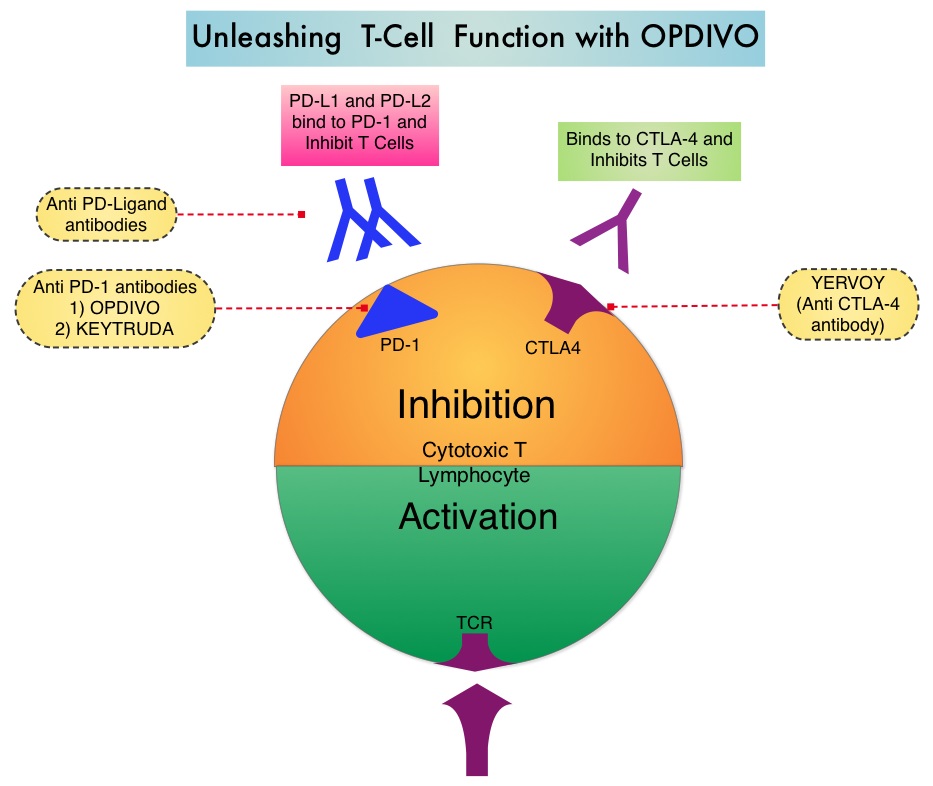
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Under normal circumstances, Immune checkpoints or gate keepers inhibit intense immune responses by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody that demonstrated significant responses in a phase 1b trial involving patients with relapsed/refractory cHL (Ansell SM et al. NEJM 2015;372:311–319).
This accelerated approval of OPDIVO® was based on two single-arm, open label, multicenter trials (CheckMate-205 and -039) of OPDIVO®, in adults with relapsed or refractory cHL. These trials enrolled patients regardless of PD-L1 expression status on Reed-Sternberg cells. The median age was 37 years and patients had a median of 5 prior therapies. The primary endpoint was Objective Response Rate (ORR) and additional outcome measures included Duration of Response (DOR). Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression or severe toxicities.
The efficacy of OPDIVO® was evaluated in 95 patients with cHL from the two single-arm trials, previously treated with autologous Hematopoietic Stem Cell Transplantation (aHSCT) and post-transplantation ADCETRIS® (Brentuximab vedotin). Patients had received a median of 17 doses of OPDIVO®. The ORR with OPDIVO® was 65% with 58% Partial Remission and 7% Complete Remission. The median time to response was 2.1 months and the estimated median DOR was 8.7 months. The most common adverse reactions (20% or more) of any grade were fatigue, upper respiratory tract infection, cough, pyrexia, and diarrhea.
The authors concluded that patients with Classical Hodgkin Lymphoma treated with OPDIVO® after autologous Hematopoietic Stem Cell Transplantation (aHSCT) and post-transplantation ADCETRIS®, have very high response rates and long Duration of Responses with acceptable toxicities. OPDIVO® is the first PD-1 inhibitor, approved for a hematologic malignancy. Checkmate 205: Nivolumab (nivo) in classical Hodgkin lymphoma (cHL) after autologous stem cell transplant (ASCT) and brentuximab vedotin (BV)—A phase 2 study. Younes A, Santoro A, Zinzani PL, et al. J Clin Oncol 34, 2016 (suppl; abstr 7535)

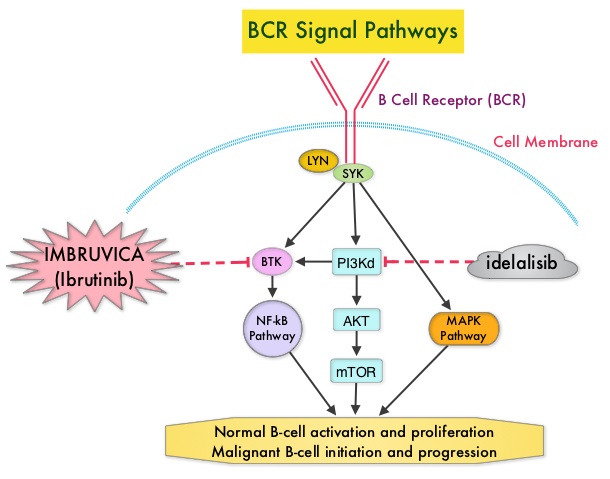
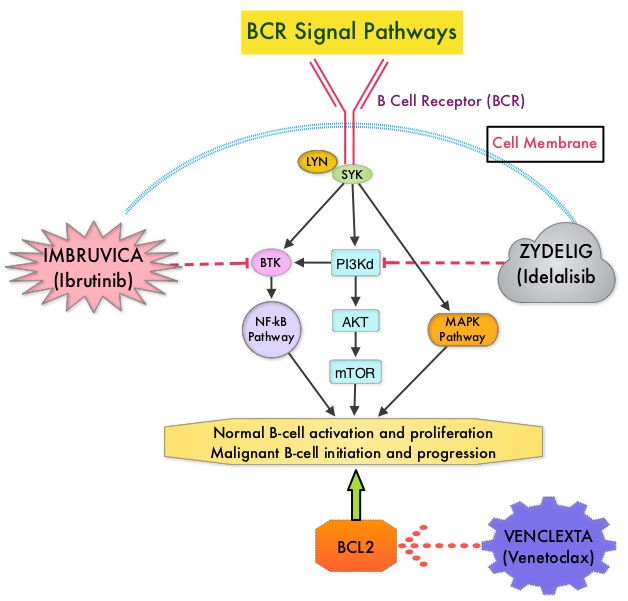 Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton's Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. PI3K (PhosphoInositide 3-Kinase) delta signaling, is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. The delta isoform of PI3K enzyme is predominantly expressed in leukocytes. Targeting proteins in key pathways of B-cell biology has fundamentally changed the management and outcomes of CLL, over the past 5 years. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. ZYDELIG® (Idelalisib) is a highly selective oral inhibitor of the enzyme PI3K and specifically blocks the delta isoform of PI3K enzyme and its signaling pathway. The pro-survival (anti-apoptotic) protein BCL2, is over expressed by CLL cells and regulates clonal selection and cell survival. A new class of anticancer agents known as BH3-mimetic drugs are in development that mimic the activity of the physiologic antagonists of BCL2 and related proteins and promote apoptosis (programmed cell death).
Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton's Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. PI3K (PhosphoInositide 3-Kinase) delta signaling, is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. The delta isoform of PI3K enzyme is predominantly expressed in leukocytes. Targeting proteins in key pathways of B-cell biology has fundamentally changed the management and outcomes of CLL, over the past 5 years. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. ZYDELIG® (Idelalisib) is a highly selective oral inhibitor of the enzyme PI3K and specifically blocks the delta isoform of PI3K enzyme and its signaling pathway. The pro-survival (anti-apoptotic) protein BCL2, is over expressed by CLL cells and regulates clonal selection and cell survival. A new class of anticancer agents known as BH3-mimetic drugs are in development that mimic the activity of the physiologic antagonists of BCL2 and related proteins and promote apoptosis (programmed cell death).