SUMMARY: VISTOGARD® (Uridine Triacetate) is presently approved in the US for the emergency treatment of adult and pediatric patients, who had severe or life-threatening toxicities within 4 days of treatment, following an overdose of 5-FluoroUracil (5-FU) or XELODA® (Capecitabine). Toxicities related to 5-FU or XELODA® can be caused by impaired drug clearance, Dihydropyrimidine Dehydrogenase deficiency, and other genetic variations in the enzymes that metabolize 5-FU. Additionally, 5-FU overdoses resulting in death can occur because of infusion pump errors, dosage miscalculations and accidental or suicidal ingestion of XELODA®. These toxicities can manifest as severe mucositis, cytopenias, central neurotoxicity and acute cardiomyopathy.
VISTOGARD® is a pyrimidine analog and following oral administration is deacetylated by nonspecific esterases, yielding Uridine in the circulation. Uridine is a direct antagonist of 5-FU and competitively inhibits 5-FU from incorporating in normal tissues, thus reducing cell damage and cell death. The authors reported the efficacy of VISTOGARD® in two open-label clinical trials in which patients who presented with a 5-FU/XELODA® overdose (N=142) or patients with early onset of severe toxicities (N=26), were treated. These patients received VISTOGARD® granules 10 grams every 6 hours for 20 doses, starting within 96 hours after the termination of 5-FU/XELODA® therapy. The median age was 58 years. Because there were no antidotes available for 5-FU toxicity at the time of this study, and the use of a placebo was unethical, the outcomes of this study were compared to a historical cohort of patients gathered from all available literature (control group), who overdosed on 5-FU and received only best supportive care. The primary endpoint of these studies was survival at 30 days or until chemotherapy could resume, if prior to 30 days.
Of the 142 overdose patients treated with VISTOGARD®,137 patients (96%) survived and had a rapid reversal of severe acute cardiotoxicity and neurotoxicity and additionally, mucositis and leukopenia were prevented, or the patients recovered from them. In the historical control cohort, 21 of 25 patients (84%) died. Among the 141 VISTOGARD® treated overdose patients with a diagnosis of cancer, 53 resumed chemotherapy in less than 30 days (median time after 5-FU of 19.6 days), indicating a rapid recovery from toxicity. The most common toxicities in patients receiving VISTOGARD® included, nausea, vomiting and diarrhea.
The authors concluded that VISTOGARD® is a safe and effective antidote for 5-FU overexposure, and can facilitate rapid recovery and resumption of chemotherapy. Patients should take VISTOGARD® as soon as possible after overdose, regardless of symptoms or within 4 days of severe or life threatening toxicity. It should be noted that VISTOGARD® is not recommended for treatment of non-emergency adverse events associated with 5-FU and XELODA®, as this therapy may significantly decrease the efficacy of these chemotherapy agents. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Ma WW, Saif WM, El-Rayes BF, et al. Cancer 2017;123:345-356.

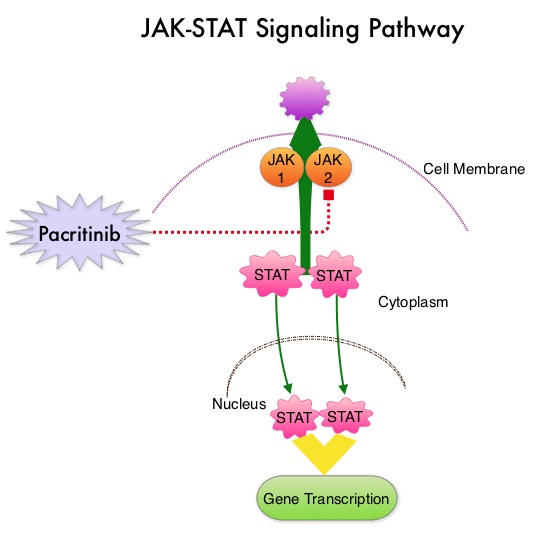
 JAKAFI® (Ruxolitinib) is a potent JAK1 and JAK2 inhibitor approved by the FDA in 2011 to treat intermediate or high-risk Myelofibrosis. It is however not indicated for patients with platelet counts under 50,000/μl, and this group represents approximately one third of Myelofibrosis patients and have limited or no treatment options. Previously published PERSIST-1 trial showed that Pacritinib significantly reduced Spleen Volume and Myelofibrosis associated symptoms, in patients with low platelet count, when compared to Best Available Therapy (excluding JAKAFI®).
JAKAFI® (Ruxolitinib) is a potent JAK1 and JAK2 inhibitor approved by the FDA in 2011 to treat intermediate or high-risk Myelofibrosis. It is however not indicated for patients with platelet counts under 50,000/μl, and this group represents approximately one third of Myelofibrosis patients and have limited or no treatment options. Previously published PERSIST-1 trial showed that Pacritinib significantly reduced Spleen Volume and Myelofibrosis associated symptoms, in patients with low platelet count, when compared to Best Available Therapy (excluding JAKAFI®).