
Written by: Thomas E Boyd, MD, Texas Oncology
Content Sponsored by: Bristol-Myers Squibb Company (BMS)
Dr. Boyd is a paid consultant for BMS and was compensated for his contribution in drafting this article.
Acute myeloid leukemia (AML) is a deadly disease with most new cases affecting patients aged 65–74 years old.1 The 5-year relative survival rate for AML is 29.5%1, highlighting a need for treatment approaches that improve survival.2 Patient- and disease-related characteristics, including medical fitness, age, cytogenetic and molecular testing, and risk of adverse events, often determine treatment options.3
After careful consideration of all factors, patients can be treated with either a higher-intensity chemotherapy option or a lower-intensity therapy option, and in some cases, additional cycles of consolidation therapy may follow the higher-intensity chemotherapy option.4 Helping patients achieve remission and keeping them there is a major goal of initial induction therapy. Continued treatment of AML in first remission may improve overall survival; however, relapse due to residual AML cells is still a major concern.2 In 764 patients with AML aged 60–85 years old who received induction therapy, ~50% relapsed within 1 year and ~80% relapsed within 5 years.5 A goal of continued treatment for AML is extending overall survival (OS) in patients who have achieved first remission.2 In some instances, extending OS can be achieved with a hematopoietic stem cell transplant; but not all patients are eligible or choose to go down this treatment route.3
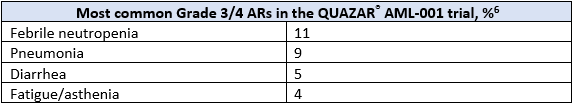
ONUREG® is the first and only FDA-approved therapy indicated for continued treatment of adult patients with acute myeloid leukemia who achieved first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) following intensive induction chemotherapy and are not able to complete intensive curative therapy.6 The efficacy and safety of ONUREG® was evaluated in the Phase 3 multicenter, randomized, double-blind placebo-controlled QUAZAR® AML-001 study.6 Eligible patients were ages 55 years or older, diagnosed with AML, were within 4 months of achieving first CR or CRi with intensive induction chemotherapy, and may have received consolidation therapy.6 Efficacy was established by OS, where ONUREG® demonstrated >2 years median OS for patients with AML in first remission as compared to placebo (24.7 months in the treatment arm vs 14.8 months in the placebo arm, hazard ratio (HR) 0.69, 95% confidence interval (CI): 0.55-0.86; P=0.0009).6 The most common adverse reactions (ARs, ≥ 10%) associated with ONUREG® treatment were nausea, vomiting, diarrhea, fatigue/asthenia, constipation, pneumonia, abdominal pain, arthralgia, decreased appetite, febrile neutropenia, dizziness, and pain in extremity.6 Serious ARs occurred in 15% of patients who received ONUREG®, and the most common Grade 3/4 ARs are shown in the table below.6
Who is an appropriate candidate for ONUREG®? The following hypothetical examples will review some of the characteristics to consider when deciding if a patient could be appropriate for ONUREG®.
Patient A is a 67-year-old retired accountant who is active and generally healthy. Their hypertension is well managed with medication, and they have no other comorbidities. Despite no family history of leukemia or hematologic abnormalities, Patient A has just been diagnosed with de novo AML not otherwise specified with intermediate-risk cytogenetics and no actionable mutations. Their hematologist prescribes intensive induction chemotherapy with the standard 7+3 regimen, and Patient A went into first remission.
Patient A is generally healthy with a well-managed comorbidity, which results in their treatment with intensive induction chemotherapy followed by first remission. Patient A is a candidate for transplant but declines one due to concerns over graft-versus-host disease. Since Patient A chose not to proceed to transplant, then ONUREG® may be an option for them.
Patient B is a 70-year retired nurse who lives alone, with family nearby. They were recently diagnosed with AML and received 7+3 chemotherapy followed by one round of consolidation. Patient B tolerated their treatment as well as could be expected and achieved first complete remission. While they are eligible for transplant, they declined and have been under observation by their doctor for the past few months. Patient B received and tolerated intensive induction chemotherapy, achieved first remission, and declined transplant, an intensive curative option. This makes them a potential candidate for continued treatment with ONUREG®.
Ultimately, the treating physician will make the final decision, but ONUREG® is indicated as a continued treatment of adult patients with AML who achieved first CR or CRi following intensive induction chemotherapy and are not able to complete intensive curative therapy.6 Understanding a patient’s disease and journey can help set them on the path where appropriate towards a continued treatment that has demonstrated an overall survival benefit in the QUAZAR® AML-001 study.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ONUREG® is contraindicated in patients with known severe hypersensitivity to azacitidine or its components.
WARNINGS AND PRECAUTIONS
Risks of Substitution with Other Azacitidine Products
Due to substantial differences in the pharmacokinetic parameters, the recommended dose and schedule for ONUREG® are different from those for the intravenous or subcutaneous azacitidine products. Treatment of patients using intravenous or subcutaneous azacitidine at the recommended dosage of ONUREG® may result in a fatal adverse reaction. Treatment with ONUREG® at the doses recommended for intravenous or subcutaneous azacitidine may not be effective. Do not substitute ONUREG® for intravenous or subcutaneous azacitidine.
Myelosuppression
New or worsening Grade 3 or 4 neutropenia and thrombocytopenia occurred in 49% and 22% of patients who received ONUREG®. Febrile neutropenia occurred in 12%. A dose reduction was required for 7% and 2% of patients due to neutropenia and thrombocytopenia. Less than 1% of patients discontinued ONUREG® due to either neutropenia or thrombocytopenia. Monitor complete blood counts and modify the dosage as recommended. Provide standard supportive care, including hematopoietic growth factors, if myelosuppression occurs.
Increased Early Mortality in Patients with Myelodysplastic Syndromes (MDS)
In AZA-MDS-003, 216 patients with red blood cell transfusion-dependent anemia and thrombocytopenia due to MDS were randomized to ONUREG® or placebo. 107 received a median of 5 cycles of ONUREG® 300 mg daily for 21 days of a 28-day cycle. Enrollment was discontinued early due to a higher incidence of early fatal and/or serious adverse reactions in the ONUREG® arm compared with placebo. The most frequent fatal adverse reaction was sepsis. Safety and effectiveness of ONUREG® for MDS have not been established. Treatment of MDS with ONUREG® is not recommended outside of controlled trials.
Embryo-Fetal Toxicity
ONUREG® can cause fetal harm when administered to a pregnant woman. Azacitidine caused fetal death and anomalies in pregnant rats via a single intraperitoneal dose less than the recommended human daily dose of oral azacitidine on a mg/m2 basis. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ONUREG® and for at least 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with ONUREG® and for at least 3 months after the last dose.
ADVERSE REACTIONS
Serious adverse reactions occurred in 15% of patients who received ONUREG®. Serious adverse reactions in ≥2% included pneumonia (8%) and febrile neutropenia (7%). One fatal adverse reaction (sepsis) occurred in a patient who received ONUREG®.
Most common (≥10%) adverse reactions with ONUREG® vs placebo were nausea (65%, 24%), vomiting (60%, 10%), diarrhea (50%, 21%), fatigue/asthenia (44%, 25%), constipation (39%, 24%), pneumonia (27%, 17%), abdominal pain (22%, 13%), arthralgia (14%, 10%), decreased appetite (13%, 6%), febrile neutropenia (12%, 8%), dizziness (11%, 9%), pain in extremity (11%, 5%).
LACTATION
There are no data regarding the presence of azacitidine in human milk or the effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with ONUREG® and for 1 week after the last dose.
Please see full Prescribing Information for ONUREG®.
References
1. National Cancer Institute. SEER Cancer Statistics Factsheets: Acute Myeloid Leukemia. http://seer.cancer.gov/statfacts/html/amyl.html. Accessed April 21, 2021.
2. Medeiros BC, Chan SM, Daver NG, Jonas BA, Pollyea DA. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am J Hematol. 2019;94:803-811.
3. Medeiros BC, Satram S. Real world treatment patterns and comparative effectiveness among elderly patients with acute myeloid leukemia in the United States. Ann Hematol Oncol. 2020;7(1):1283.
4. Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487-494.
5. Büchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61-69.
6. ONUREG®® [Prescribing Information]. Summit, NJ: Celgene Corporation; 2021.
© 2021 Celgene Corporation.
ONUREG® is a trademark of Celgene Corporation, a Bristol-Myers Squibb company.
QUAZAR® is a registered trademark Celgene Corporation, a Bristol-Myers Squibb company.
12/21 2011-US-2100198

