SUMMARY: The American Society of Clinical Oncology (ASCO) recently provided recommendations for the prophylaxis and treatment of venous thromboembolism (VTE) in patients with cancer following a systematic review of 42 publications. They are as follows-
VTE prophylaxis in hospitalized patients
Majority of the hospitalized patients with active malignancy should receive VTE prophylaxis in the absence of contraindications. However there are no definitive data for VTE prophylaxis in patients admitted for short chemotherapy infusions, minor procedures or those undergoing stem-cell or bone marrow transplantation.
VTE prophylaxis in ambulatory patients
Routine VTE prophylaxis is not recommended although prophylaxis with low molecular-weight heparin can be considered in select group of outpatients with solid tumors who are receiving chemotherapy, after discussing the risk/benefits of such intervention. Patients with Myeloma receiving THALOMID® (Thalidomide) or REVLIMID® (Lenalidomide) should receive VTE prophylaxis with either aspirin or low molecular-weight heparin.
VTE prophylaxis in patients undergoing surgery
All cancer patients undergoing major surgical procedures should be considered for VTE prophylaxis with heparin unless there is a bleeding risk. This should be commenced preoperatively and can be complemented with mechanical methods to improve efficacy. This should be continued for at least 7-10 days and for up to 4 weeks postoperatively, in those undergoing major abdominal or pelvic surgery with restricted mobility, obesity, history of VTE or additional risk factors. Decision to consider prophylaxis, for patients undergoing low risk surgery, should be made on an individual basis.
Choice of Anticoagulation for cancer patients with VTE
For cancer patients who have a creatinine clearance of more than 30 ml/min and have newly diagnosed VTE , low molecular-weight heparin is preferred over unfractionated heparin for the initial 5 to 10 days. Low molecular-weight heparin, due to its superiority, is preferred over vitamin K antagonists for long term anticoagulation (6 months). Select cancer patients with metastatic disease or those receiving chemotherapy may be considered for anticoagulation with low molecular-weight heparin or vitamin K antagonists beyond the initial 6 months. A vena cava filter is indicated only for patients with contraindications to anticoagulant therapy and may be considered for those who progress despite optimal anticoagulation with low molecular-weight heparin. Patients with CNS malignancies and VTE should be anticoagulated like any other patient with VTE, monitoring closely for bleeding. Incidental VTE’s should be treated like symptomatic VTE. Decision to treat splanchnic or visceral vein thrombi diagnosed incidentally should be considered on an individual basis. The new novel oral anticoagulants are presently not recommended for prophylaxis or treatment of VTE.
Anticoagulation and survival benefit in patients without VTE
Cancer patients without VTE should not receive anticoagulants to improve survival, as there are no data to support this recommendation.
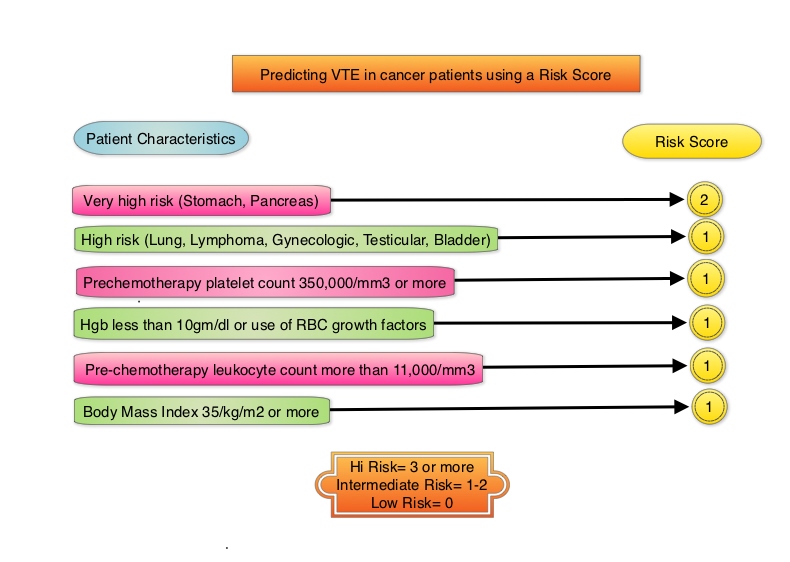
Predicting VTE in cancer patients using Risk Score
The risk score model below for VTE in cancer patients was developed and validated in multiple studies. The risk of VTE can be significantly reduced amongst these high risk patients by thromboprophylaxis
Lyman GH, Khorana AA, Kuderer NM, et al. J Clin Oncol 2013;31:2189-2204