SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Small cell lung cancer (SCLC) accounts for approximately 13-15% of all lung cancers and is aggressive. Patients with extensive stage SCLC are often treated with a combination of Carboplatin or Cisplatin with Etoposide as first line treatment and the tumor response rates are as high as 60-80%. However, majority of the patients relapse within months of completing initial therapy, with a median Overall Survival of approximately 10 months. Based on the premise that SCLC has a high mutation rate, it was hypothesized that these tumors may be immunogenic and could respond to immune-checkpoint inhibitors. This hypothesis was subsequently confirmed in patients with refractory or metastatic SCLC.
TECENTRIQ® (Atezolizumab) is an anti PD-L1 monoclonal antibody that directly binds to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells and blocks the interaction of PD-L1 with PD-1 and B7.1 receptors and thereby enables the activation of T cells and restores tumor-specific T-cell immunity. In a phase I trial, TECENTRIQ® monotherapy demonstrated durable responses, with an acceptable safety profile in patients with relapsed or refractory SCLC. The authors in this study combined checkpoint inhibition with cytotoxic chemotherapy for synergy and improved efficacy. 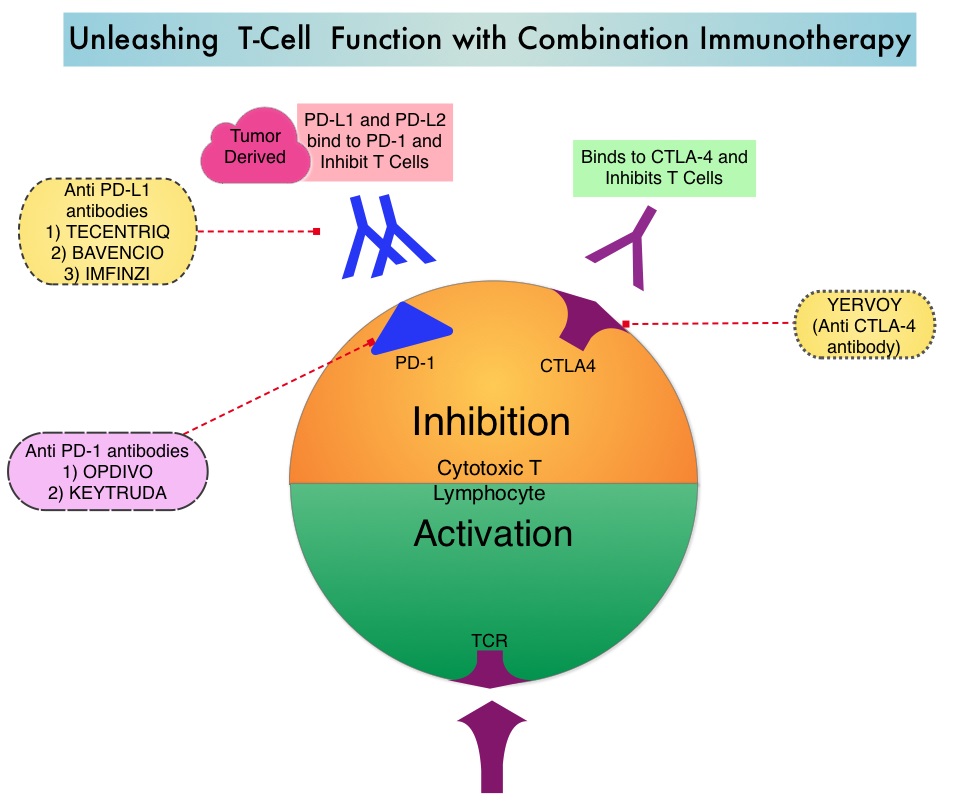
The IMpower133 trial is a multinational, randomized, double-blind, placebo-controlled phase III trial which evaluated the benefit of TECENTRIQ® plus Carboplatin and Etoposide in chemo naïve patients with extensive-stage Small-Cell Lung Cancer. Enrolled patients were randomized in a 1:1 ratio and the induction phase consisted of four cycles of Carboplatin AUC 5 mg/ml/min IV on day 1 and Etoposide 100 mg/m2 IV on days 1-3 of each 21-day cycle, with either TECENTRIQ® 1200 mg IV day 1 of each cycle (N=201) or placebo (N=202). The induction phase was followed by a maintenance phase during which patients received either TECENTRIQ® or placebo (based on previous random assignment) and treatment was continued until disease progression or unacceptable toxicities. The median age was 64 yrs and PD-L1 testing was not a requirement. Prophylactic cranial irradiation was permitted during the maintenance phase of treatment but thoracic radiation therapy was not permitted. The Primary end points were Overall Survival (OS) and Progression Free Survival and Secondary end points included Objective Response Rate (ORR) and Duration of Response.
At a median follow up of 13.9 months, the median OS was 12.3 months in the TECENTRIQ® group compared to 10.3 months in the placebo group (HR=0.70; P=0.007). This suggested a 30% reduction in the risk of death when TECENTRIQ® was added to chemotherapy. The 1-year OS rate was 51.7% in the TECENTRIQ® group and 38.2% in the placebo group. The median Progression Free Survival was also longer in the TECENTRIQ® group than in the placebo group (5.2 months versus 4.3 months, HR=0.77; P=0.02). Survival benefits were consistently seen across patient subgroups. The safety profile of TECENTRIQ® plus Carboplatin and Etoposide was consistent with the previously reported safety profile of the individual agents, with no new findings observed in this trial.
It was concluded that in this multinational trial, the addition of TECENTRIQ® to chemotherapy in the first line treatment of extensive stage Small-Cell Lung Cancer resulted in significantly longer Overall Survival and Progression Free Survival, when compared to chemotherapy alone. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. Horn L, Mansfield AS, SzczÄ™sna A, et al. for the IMpower133 Study Group. N Engl J Med 2018; 379:2220-2229

