SUMMARY: Chronic Myeloid Leukemia (CML) constitutes a little over 10% of all new cases of leukemia. The American Cancer Society estimates that about 8,220 new CML cases will be diagnosed in the United States in 2016 and about 1,070 patients will die of the disease. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation between chromosomes 9 and 22, wherein the ABL gene from chromosome 9 fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells.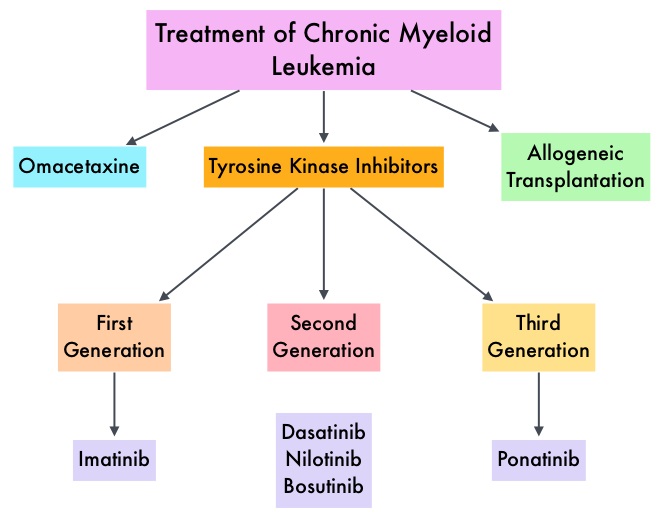 The presently available Tyrosine Kinase Inhibitors (TKI’s) approved in the United States including GLEEVEC®, share the same therapeutic target, which is BCR-ABL kinase. Resistance to TKI’s can occur as a result of mutations in the BCR-ABL kinase domain or amplification of the BCR-ABL gene. With the availability of newer therapies for CML, monitoring response to treatment is important. This is best accomplished by measuring the amount of residual disease using Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Molecular response in CML is expressed using the International Scale (IS) as BCR-ABL%, which is the ratio between BCR-ABL and a control gene. BCR-ABL kinase domain point mutations are detected, using the mutational analysis by Sanger sequencing. Majority of the patients receiving a TKI following diagnosis of CML achieve a Complete Cytogenetic Response (CCyR) within 12 months following commencement of therapy and these patients have a life expectancy similar to that of their healthy counterparts. Previously published studies have shown that deep molecular response (MR4.5) is a new molecular predictor of long term survival in CML patients and was achieved in a majority of patients treated with optimized dose of GLEEVEC®. It has been hypothesized based on previous observations, that a subgroup of CML patients experiencing deeper responses (MR3, MR4, and MR4.5), may stay in unmaintained remission even after treatment discontinuation. Despite this observation, stopping CML therapy is currently not a clinical recommendation and should only be considered in the context of a clinical trial.
The presently available Tyrosine Kinase Inhibitors (TKI’s) approved in the United States including GLEEVEC®, share the same therapeutic target, which is BCR-ABL kinase. Resistance to TKI’s can occur as a result of mutations in the BCR-ABL kinase domain or amplification of the BCR-ABL gene. With the availability of newer therapies for CML, monitoring response to treatment is important. This is best accomplished by measuring the amount of residual disease using Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Molecular response in CML is expressed using the International Scale (IS) as BCR-ABL%, which is the ratio between BCR-ABL and a control gene. BCR-ABL kinase domain point mutations are detected, using the mutational analysis by Sanger sequencing. Majority of the patients receiving a TKI following diagnosis of CML achieve a Complete Cytogenetic Response (CCyR) within 12 months following commencement of therapy and these patients have a life expectancy similar to that of their healthy counterparts. Previously published studies have shown that deep molecular response (MR4.5) is a new molecular predictor of long term survival in CML patients and was achieved in a majority of patients treated with optimized dose of GLEEVEC®. It has been hypothesized based on previous observations, that a subgroup of CML patients experiencing deeper responses (MR3, MR4, and MR4.5), may stay in unmaintained remission even after treatment discontinuation. Despite this observation, stopping CML therapy is currently not a clinical recommendation and should only be considered in the context of a clinical trial.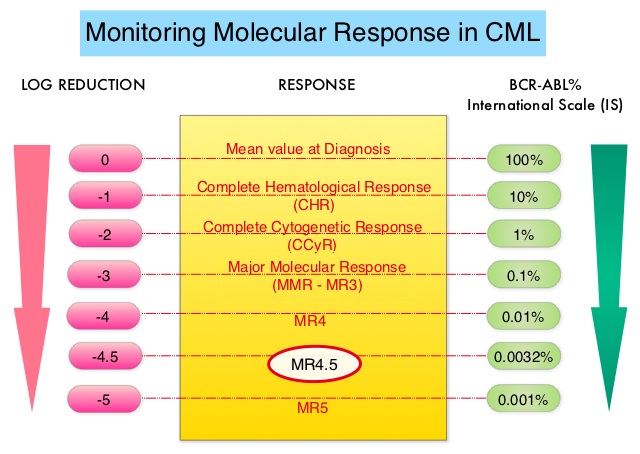
ENESTfreedom (Evaluating Nilotinib Efficacy and Safety in Clinical Trial – Following REsponsE in De nOvo CML-CP Patients) is a single arm, open-label, international, multicenter phase II study, which evaluated Treatment-Free Remission (TFR) of patients with CML-Chronic Phase, after stopping TASIGNA® (Nilotinib), following frontline treatment with this agent. This analysis included 215 patients Philadelphia Chromosome positive (Ph+) CML patients in the Chronic Phase, who received at least 2 years of frontline treatment with TASIGNA® and had achieved a Molecular Response 4.5 (BCR-ABL International Scale 0.0032%). Patients then continued TASIGNA® for an additional year (Consolidation Phase) with close monitoring for sustained deep molecular response using real-time quantitative PCR assessments at 12-week intervals. Patients who had a molecular response no worse than MR4 (0.01% or less) in the last assessment of the Consolidation phase (N=190), were then eligible to stop treatment (TFR phase). The median age of these patients was 55 years. The median time from study entry to first MR4.5 was 18 months. The median duration of treatment on TASIGNA® prior to TFR was 43 months.
After 48 weeks without treatment, 51.6% of the 190 patients in the TFR phase remained in MMR and did not require re-initiation of treatment. Of the 86 patients who restarted treatment with TASIGNA® due to loss of MMR, 99% were able to regain MMR and 88% were able to regain MR4.5. The median time to MMR for these patients was 7.9 weeks and for MR4.5 was 13.1 weeks. About 25% of the patients experienced musculoskeletal pain during the first year of the TFR phase, which is an expected adverse event from TKI withdrawal.
The authors concluded that TFR is clinically meaningful and a high rate of sustained MR4.5 leads to stable disease. TASIGNA® is the first Tyrosine Kinase Inhibitor to demonstrate successful TFR in a large proportion of eligible patients after relatively short duration of treatment of 3.6 years. Over 50% of patients who stopped therapy were able to remain treatment free at 48 weeks. The authors recommended that frequent patient monitoring during Treatment-Free Remission (TFR) period, allows timely determination of loss of MR4.0 and MMR and the need for treatment re-initiation. Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study. Hochhaus A, Masszi T, Giles FJ, et al. J Clin Oncol 34, 2016 (suppl; abstr 7001)

