SUMMARY: Chronic Myeloid Leukemia (CML) constitutes about 15% of all new cases of leukemia. The American Cancer Society estimates that about 9,280 new CML cases will be diagnosed in the United States in 2024 and about 1,280 patients will die of the disease. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation between chromosomes 9 and 22, wherein the ABL gene from chromosome 9 fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells.
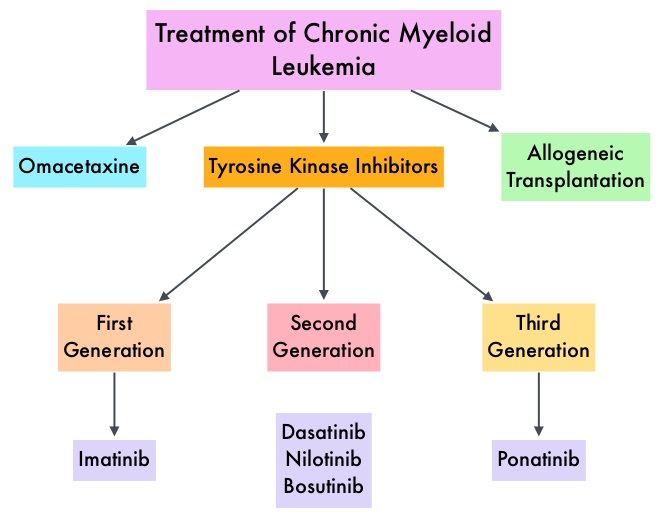
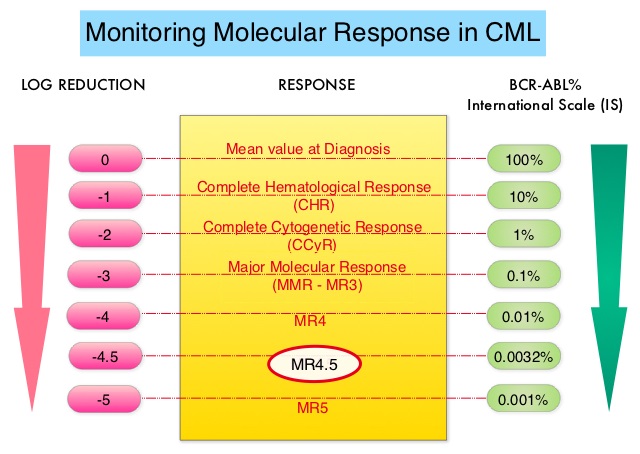
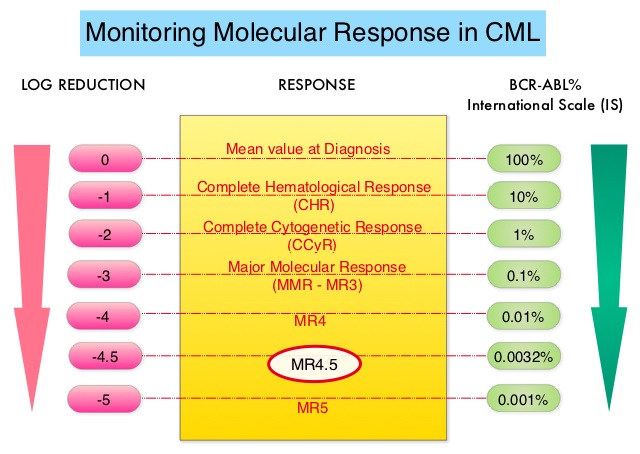
Chronic Myeloid Leukemia has long been a model for targeted cancer therapy, particularly through the development of Tyrosine Kinase Inhibitors (TKIs) targeting the BCR:ABL1 fusion gene. The presently available Tyrosine Kinase Inhibitors (TKI’s) approved in the United States including Imatinib, share the same therapeutic target, which is BCR-ABL kinase. Resistance to TKI’s can occur as a result of mutations in the BCR-ABL kinase domain or amplification of the BCR-ABL gene. With the availability of newer therapies for CML, monitoring response to treatment is important. This is best accomplished by measuring the amount of residual disease using Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Molecular response in CML is expressed using the International Scale (IS) as BCR-ABL%, which is the ratio between BCR-ABL and a control gene. BCR-ABL kinase domain point mutations are detected using the mutational analysis by Sanger sequencing. Majority of the patients receiving a TKI following diagnosis of CML achieve a Complete Cytogenetic Response (CCyR) within 12 months following commencement of therapy and these patients have a life expectancy similar to that of their healthy counterparts. Previously published studies have shown that Deep Molecular Response (BCR-ABL <0.01% on the International Scale-MR4) is a new molecular predictor of long term survival in CML patients, and this was achieved in a majority of patients treated with TKIs. Further, it has been shown from previous observations, that a subgroup of CML patients experiencing deeper responses (MR3, MR4, and MR4.5), may stay in unmaintained remission even after treatment discontinuation. Despite this observation, precise criteria for stopping CML therapy have not been clearly defined.
Discontinuing TKI therapy after a Deep Molecular Response among patients with CML can potentially improve quality of life, minimize long term toxicities as well as drug-drug interactions, and reduce financial burden. Stopping TKI therapy among CML patients appears to be safe and feasible in over 50% of the patients, although about 20% of these patients experience TKI withdrawal syndrome manifesting as musculoskeletal symptoms. Discontinuation of TKI therapy should only be considered in consenting patients after a thorough discussion of the potential risks and benefits. TKIs have revolutionized the prognosis and quality of life for patients with CML, leading to a new treatment goal of achieving Treatment-Free Remission (TFR).
The European Stop Kinase Inhibitors (EURO-SKI) study is the largest clinical trial conducted to assess the safety of stopping Tyrosine Kinase Inhibitor therapy in patients with CML, whose leukemia was in stable Deep Molecular Response (DMR). The researchers presented the final analysis of the EURO-SKI trial after 3 years of follow up and highlighted the prognostic factors for short- and long-term molecular response maintenance. This comprehensive study evaluated the effects of stopping TKI treatment (Imatinib, Nilotinib or Dasatinib), in patients who had been on therapy for at least 3 years and had confirmed DMR, defined as BCR:ABL1-transcripts 0.01% or less on the International Scale for at least 12 months. The Primary outcomes of the study were the maintenance of Major Molecular Response (MMR), defined as BCR:ABL1 0.1% or less (MR3), at 6 and 36 months after stopping TKIs (Molecular Recurrence Free Survival).
In this study, 868 patients with Chronic Phase CML were screened, and 728 patients were included in the baseline analysis. The final analysis revealed that 61% of patients remained in MMR at 6 months, and 46% remained in MMR at 36 months after stopping TKI treatment. Several factors were identified as significant predictors of MMR maintenance. Longer duration of TKI treatment and DMR before stopping TKI treatment were associated with a higher likelihood of maintaining MMR at 6 months. Additionally, the type of BCR:ABL1 transcript emerged as a prognostic factor, with patients having transcript type e14a2 alone or in combination with e13a2 showing a significantly higher probability of maintaining MMR. For MMR maintenance between 6 and 36 months, the duration of TKI treatment (but not DMR duration) before stopping TKI treatment, and disease characteristics at diagnosis, including percentage of peripheral blood blast cells and platelet count at diagnosis, were significant factors influencing MMR maintenance. Among 315 patients evaluated at 36 months, the Molecular Recurrence Free Survival was 76%. Multivariate analysis over the entire 36-month trial period identified duration of TKI treatment, duration of DMR (Deep Molecular Response) while receiving TKI, percentage of peripheral blood blast cells at diagnosis, and transcript type (e14a2 plus e13a2 had a higher probability of maintaining MMR over 36 months than e13a2 alone) as independent factors for MMR maintenance.
The findings of the EURO-SKI trial have important implications and represent a significant milestone for the management of CML. They highlight the importance of considering not only the duration of TKI treatment but also disease characteristics and transcript type when predicting treatment-free remission. This study represents a significant step forward in understanding the factors influencing Treatment-Free Remission in CML patients, and may help guide clinical decision-making in the future.
European Stop Tyrosine Kinase Inhibitor Trial (EURO-SKI) in Chronic Myeloid Leukemia: Final Analysis and Novel Prognostic Factors for Treatment-Free Remission. Mahon F-X, Pfirrmann M, Dulucq S, et al. on behalf of the EURO-SKI Investigators. Journal of Clinical Oncology. March 12, 2024. https://doi.org/10.1200/JCO.23.01647.

 This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241).
This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241).