SUMMARY: Indolent Non-Follicular B-Cell Lymphoma (INFBCL) are mature B cell lymphoproliferative disorders and include Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT) lymphoma, Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL).
NMZL, LPL, and SLL
Recommendations for Diagnosis:
1) Excision biopsy of material from the primary disease site (ie, lymph node)
2) Fine Needle Aspiration biopsy is not recommended for the diagnosis or sub-typing. Computed Tomography (CT)-guided core biopsy can be an alternative diagnostic approach when thoracotomy or laparotomy are needed for lymph node biopsy.
3) A definitive diagnosis of LPL can only be made using bone marrow material.
Recommendations for Staging and Pretreatment Evaluation:
1) Complete history and physical examination, assessing Performance Status and B symptoms
2) Lab tests including CBC, CMP, LDH, B2-Microglobulin, hemolysis workup if anemic, SPEP, UPEP with immunofixation, serology for Hepatitis B,C and HIV, Flow Cytometry if peripheral blood shows abnormal lymphocytes or absolute lymphocytosis present and bone marrow aspirate and biopsy
3) CT of the neck, chest, abdomen, and pelvis (FDG-PET is not routinely indicated for this group of lymphomas as the avidity of FDG uptake is lower)
4) In LPL with monoclonal protein in the urine or serum, a fat pad biopsy for Congo red staining and an ultrasound cardiac scan are recommended when Amyloidosis is suspected
5) ECHO or MUGA scan, if treatment with anthracyclines are planned
6) Pregnancy testing in women of child-bearing age and counseling for the preservation of fertility
Recommendations regarding treatment initiation
1) Initiation of Chemotherapy or Immunotherapy should be based on the identification of symptoms as defined by the GELF or BNLI criteria.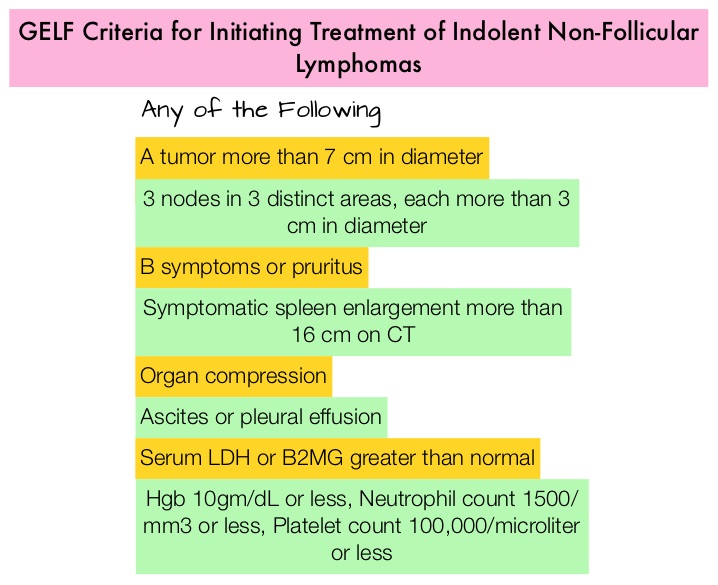
2) Monoclonal protein-related symptoms such as hyperviscosity syndrome, renal failure, vasculitis and a lymphocyte doubling time of less than 6 months in those with leukemia, are indications for treatment.
3) Chemoimmunotherapy or Chemotherapy alone as early treatment is not recommended in asymptomatic advanced disease and observation alone is recommended in patients who do not meet the criteria for treatment with Chemotherapy or Immunotherapy
Recommendations for First Line Therapy
1) Patients with stage I or nonbulky stage II disease should be promptly treated with involved field Radiotherapy (RT) with or without chemotherapy. Observation alone may be a reasonable alternative if the potential toxicity of RT outweighs the potential benefits, particularly in elderly patients or if the patient refuses RT.
2) FLUDARA® (Fludarabine) plus RITUXAN® (Rituximab) – FR or TREANDA® (Bendamustine) plus RITUXAN® (BR) is the recommended first line therapy.
3) The combination of FLUDARA®, Cyclophosphamide and RITUXAN® (FCR) should be recommended to patients less than 70 years of age without significant comorbidities, and TREANDA® plus RITUXAN® should be preferred for patients 70 years or older with significant comorbidities.
Recommendations for Postinduction Therapy
Maintenance RITUXAN® is not recommended and patients with a partial response (PR) with no symptoms or signs of active disease, should be observed without additional treatment until progression.
Recommendations for Patients with Relapse
BR combination is the preferred treatment and new combinations of RITUXAN® with REVLIMID® (Lenalidomide) or VELCADE® (Bortezomib) should be considered experimental
ENMZL of MALT Lymphoma of Gastric Mucosa (Gastric MALToma)
MALT lymphoma histology is the most frequent among Marginal Zone Lymphomas and the most common extranodal MALT lymphoma involves the gastrointestinal tract.
Recommendations for Diagnosis:
1) Biopsy of material from the primary disease site
2) A molecular genetic analysis of lymphoma tissue for the detection of t(11;18) is recommended to identify disease that is unlikely to respond to antibiotic therapy
3) The presence of active Helicobacter pylori (Hp) infection must be determined using histologic testing. In the case of negative results, serology testing, stool antigen testing, or the urea breath test is recommended
Recommendations for Staging and Pretreatment Evaluation:
1) A detailed description of the extent of gastric lesions, gastric wall infiltration, and involvement of the perigastric lymph nodes, with ultrasound-guided endoscopy.
2) Along with the conventional staging procedures, additional investigations should include CT of the neck, chest, abdomen and pelvis and bone marrow biopsy
3) Staging classification should use the Ann Arbor staging system modified according to the Paris staging system
Recommendations for First Line Therapy
1) The first-line treatment of Hp-positive patients with gastric MALT lymphoma is Hp eradication therapy, independent of the disease stage. Surgery should only be considered for patients with perforation or bleeding, not amenable to endoscopy
2) Patients with Hp-negative localized gastric MALT lymphoma can also be treated with eradication therapy although the chance of a response is low and disease status should be closely monitored during therapy
3) After successful Hp eradication, consolidation chemotherapy is not indicated
Recommendations for non-responding and relapsed Patients
1) Radiation therapy is recommended for patients with stage IE-IIE gastric MALT lymphoma
2) RITUXAN® plus chemotherapy is recommended for those with other disease stages
SMZL (Splenic Lymphoma With Circulating Villous Lymphocytes)
Recommendations for Diagnosis:
1) SMZL can be diagnosed by splenectomy and examination of the splenic tissue
2) SMZL can also be diagnosed by a combination of bone marrow biopsy and an immunocytochemistry profile (intrasinusoidal infiltration by CD20+ cells), peripheral blood and bone marrow aspirate morphology as well as flow cytometry.
Recommendations for Staging and Pretreatment Evaluation:
1) Complete history and physical examination assessing performance status and B symptoms
2) Lab tests should include CBC, CMP, LDH, B2-Microglobulin, hemolysis workup if anemic, SPEP, UPEP with immunofixation, cryoglobulin and cryocrit. Serology for Hepatitis B,C and HIV, flow cytometry if peripheral blood shows abnormal lymphocytes or absolute lymphocytosis present and bone marrow aspirate and biopsy
3) CT of the neck, chest, abdomen, and pelvis (FDG-PET is not routinely indicated for this group of lymphomas as the avidity of FDG uptake is lower)
4) Pregnancy testing in women of child-bearing age and counseling for the preservation of fertility
Recommendations regarding treatment initiation
Patients with SMZL should be initiated on therapy if there is progressive or symptomatic splenomegaly, hemoglobin less than 10 g/dL, neutrophils less than 1000/μL, progressive thrombocytopenia, systemic symptoms, progressive nodal disease and autoimmune hemolytic anemia.
Recommendations for First Line Therapy
1) Patients who are HCV positive with no indications for anti-lymphoma therapy, should be treated for HCV infection.
2) For those requiring therapy for lymphoma, the options include splenectomy, chemotherapy alone, RITUXAN® alone, or chemoimmunotherapy. Chemoimmunotherapy is indicated for good PS patients with disseminated disease. Combinations therapies with proven efficacy include RITUXAN® in combination with Chlorambucil, CVP (Cyclophosphamide, Vincristine, Prednisone), FLUDARA® or 2-CDA (Cladribine).
3) Splenectomy should be recommended when patients present with splenomegaly-related cytopenias in the absence of a high percentage of leukemic cells in the peripheral blood, heavy bone marrow infiltration, and diffuse nodal disease. In patients who are HCV positive, splenectomy should be considered only after the exclusion of a severe chronic liver disease.
4) Single agent RITUXAN® may be considered for patients without disseminated disease and for patients with contraindications to surgery or chemoimmunotherapy.
Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation Guidelines for the Management of Indolent, Nonfollicular B-Cell Lymphoma (Marginal Zone, Lymphoplasmacytic, and Small Lymphocytic Lymphoma). Tarella C, Arcaini L, Baldini L, et al. Clinical Lymphoma, Myeloma & Leukemia. 2015; 15:75–85

