SUMMARY: It is estimated that in the US, approximately 91,270 new cases of melanoma will be diagnosed in 2018 and about 9,320 patients are expected to die of the disease. The incidence of melanoma has been on the rise for the past three decades. Brain metastases are a frequent complication of solid tumors and these patients tend to have a very poor prognosis with a median survival of a few weeks to months. Malignant melanoma has the highest propensity to metastasize to the brain. More than one third of patients with advanced melanoma have brain metastases at the time of diagnosis, and up to 75% of patients have brain metastases at the time of death. Prognosis of patients with melanoma who have brain metastases is poor, with a median Overall Survival of 4-5 months and a 5 year survival of 5%. This is because systemic chemotherapy has minimal antitumor activity in the brain, does not decrease the risk of development of new brain metastases, does not control of extracranial disease and does not improve Overall Survival.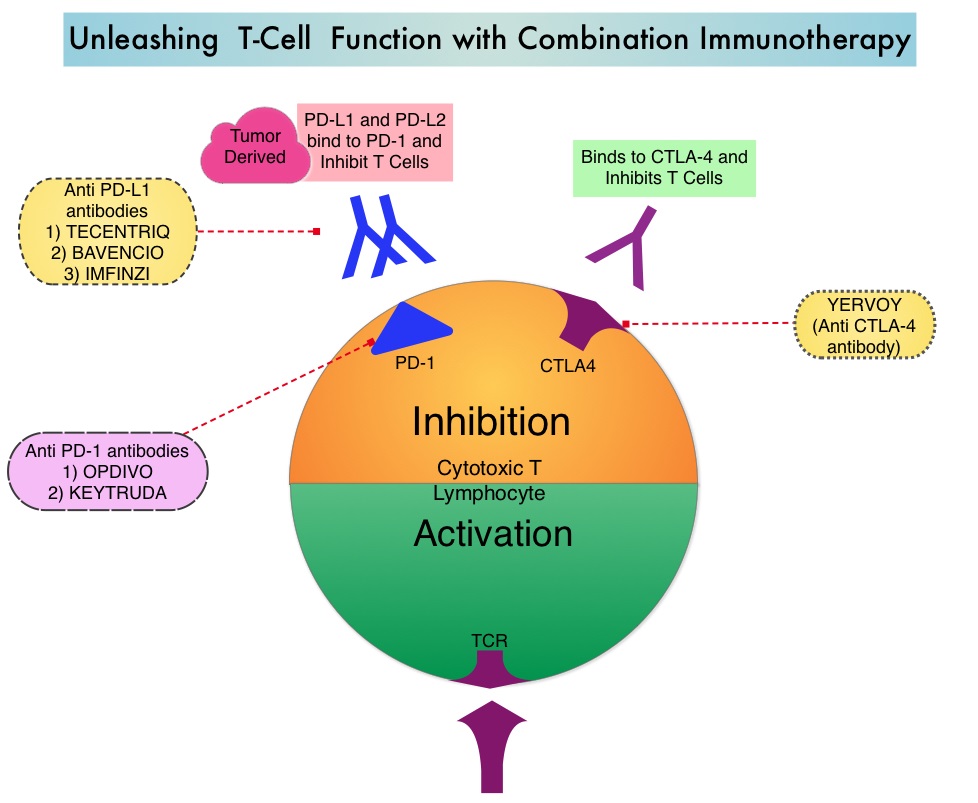
Immune checkpoint inhibitors by blocking immune checkpoint proteins unleash T cells, resulting in T cell proliferation, activation and a therapeutic response. YERVOY® (Ipilimumab) is a fully human immunoglobulin G1 monoclonal antibody that blocks immune checkpoint protein/receptor CTLA-4, and has been shown to have activity against brain metastases from melanoma when used individually as monotherapy. OPDIVO® (Nivolumab) is a fully human, Immunoglobulin G4, anti PD-1 targeted monoclonal antibody. It binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, following which the tumor-specific effector T cells are unleashed. OPDIVO® when combined with YERVOY® significantly improved Overall Survival in patients with previously untreated advanced melanoma, compared with YERVOY® alone, in phase II and III studies. These studies however excluded patients with untreated brain metastases.
CheckMate 204 is an open-label, multicenter, phase II study, conducted at 28 sites in the United States and the authors in this study evaluated the efficacy and safety of OPDIVO® plus YERVOY® in patients with melanoma who had untreated brain metastases. This study enrolled 101 patients with metastatic melanoma and at least one measurable, nonirradiated brain metastasis (tumor diameter 0.5-3 cm) and no neurologic symptoms. Patients received OPDIVO® 1 mg/kg plus YERVOY® 3 mg/kg every 3 weeks for up to four doses, followed by OPDIVO® 3 mg/kg every 2 weeks until progression or unacceptable toxic effects. The median age was 59 years, 44% of patients had PD-L1 expression of 1% or more, 22% of the patients had 3 or more target lesions and 17% had received previous systemic anticancer therapy, with BRAF inhibitor being the most common (11%), a MEK inhibitor (9%), or both. Patients with known leptomeningeal involvement, those with metastases larger than 3 cm in diameter, and those patients receiving glucocorticoid therapy, were excluded from the study. The Primary end point was the rate of intracranial clinical benefit, defined as the percentage of patients who had stable disease for at least 6 months, Complete Response, or Partial Response. Of the 101 patients enrolled, 94 patients had a minimum follow up of 6 months (median follow up 14.0 months), and could be evaluated for the Primary end point.
With a median follow up of 14 months, the rate of intracranial clinical benefit was 57%, with a Complete Response rate of 26%, Partial Response rate of 30%, and 2% of the patients had stable disease for at least 6 months. Similar rates of Objective Response Rate (50%) and Clinical Benefit (56%) were observed for extracranial lesions. The median time to intracranial response was 2.3 months. The safety profile of the regimen was similar to that reported in patients with melanoma who did not have brain metastases, and treatment-related grade 3/ 4 Adverse Events were reported in 55% of patients.
It was concluded that OPDIVO® combined with YERVOY® is an effective treatment for metastatic melanoma patients with asymptomatic, untreated brain metastases. In this study, patients had clinically meaningful intracranial efficacy, concordant with extracranial activity. The safety profile in this population was similar to that reported in studies involving patients without brain metastases. This regimen should be considered as first-line therapy for all eligible metastatic melanoma patients, with brain metastases. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. Tawbi HA, Forsyth PA, Algazi A, et al. N Engl J Med 2018; 379:789-790

