SUMMARY: Chronic Myeloid Leukemia (CML) constitutes a little over 10% of all new cases of leukemia. The American Cancer Society estimates that about 8,220 new CML cases will be diagnosed in the United States in 2016 and about 1,070 patients will die of the disease. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation-t(9;22), between chromosomes 9 and 22, wherein the ABL gene from chromosome 9, fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells. Even though the reciprocal translocation resulting in the formation of Philadelphia chromosome involves a fairly constant breakpoint in the ABL gene on chromosome 9, the breakpoint in the BCR gene on chromosome 22 can vary, resulting in different BCR-ABL transcript types. There has been ongoing debate whether the type of transcript, has prognostic significance, for patients with newly diagnosed chronic phase CML. Over 95% of patients with CML have expression of e13a2 (b2a2), e14a2 (b3a2), or both transcripts, coding for p210 BCR-ABL tyrosine kinase, whereas a small minority of patients express rare variants such as e1a2 transcripts, which code for p190 BCR-ABL, which is associated with aggressive disease. Previously published studies have shown that expression of certain type of transcripts may predict response to therapy, as well as outcomes.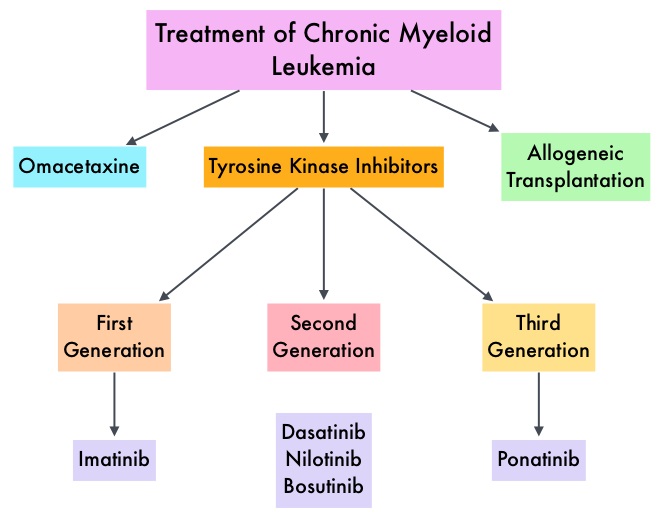
The purpose of this study was to evaluate the prognostic relevance of the commonly expressed BCR-ABL transcripts in patients with chronic phase CML and the influence of the transcript type, on molecular and cytogenetic responses, across chronic phase CML patients, treated with different Tyrosine Kinase Inhibitors (TKI). This analysis included 481 treatment naïve patients with chronic phase CML treated with different TKI modalities, expressing e13a2 (42%), e14a2 (41%), or coexpression of both e13a2 with e14a2 (18%) transcripts. These patients were treated with 4 different frontline TKIs which included, GLEEVEC® (Imatinib) 400 mg daily (N=69), GLEEVEC® 800 mg daily (N=199), SPRYCEL® (Dasatinib) 50 mg twice daily or 100 mg daily (N=105) and TASIGNA® (Nilotinib) 400 mg twice daily (N=108).
It was noted that patients with e13a2 transcripts who received GLEEVEC® 400 mg had an inferior Complete Cytogenetic Response (77%) compared with other TKI modalities (90-95%). Unlike these patients, patients with e14a2 transcripts or those expressing both e13a2 and e14a2 transcripts treated with GLEEVEC® 400 mg, had a Complete Cytogenetic Response rate of 93%, which was similar to treatment with other TKI modalities (93-96%). Even though the time to Complete Cytogenetic Response was 3 months and similar in all treatment groups, the trend for lower rates of Complete Cytogenetic Response and Major Cytogenetic Response for the e13a2 transcript cohort compared with the e14a2 cohort, persisted even at 60 months. Patients with e13a2 treated with GLEEVEC® 400 mg similarly had an inferior Major Molecular Response (MMR) at all time points than individuals with e14a2 and inferior MR4.5, compared with those treated with other TKI modalities. In patients with e14a2 transcripts, the MMR and MR4.5 rates were generally similar with all TKI modalities. In a multivariate analysis, patients with e14a2 alone or those with coexpressed e13a2, achieved an earlier and deeper response, compared to those with e13a2 transcripts, and predicted for longer event-free and transformation-free survival.
The authors concluded that the type of BCR-ABL transcript may determine outcomes in patients with chronic phase CML. Patients with e13a2 transcripts have lower platelet count and inferior outcomes with GLEEVEC® 400 mg, whereas patients with e14a2 have favorable outcomes regardless of TKI treatment modality. Further, expression of e14a2 or both e14a2 and e13a2 predicts optimal responses and longer Event Free Survival and Transformation Free Survival. Thus BCR-ABL transcript type may help in selecting the appropriate treatment and may predict outcomes in patients with chronic phase CML. Impact of BCR-ABL transcript type on outcome in patients with chronic-phase CML treated with tyrosine kinase inhibitors. Jain P, Kantarjian H, Patel KP, et al. Blood 2016;127:1269-1275

