SUMMARY: The FDA on February 15, 2019, approved KEYTRUDA® (Pembrolizumab) for the adjuvant treatment of patients with Melanoma with involvement of lymph node(s) following complete resection. It is estimated that in the US, approximately 96,480 new cases of Melanoma will be diagnosed in 2019 and about 7,230 patients are expected to die of the disease. The incidence of Melanoma has been on the rise for the past three decades. Surgical resection with a curative intent is the standard of care for patients with early stage Melanoma, with a 5-year survival rate of 98% for stage I disease and 90% for stage II disease. Stage III malignant Melanoma however is a heterogeneous disease, and the risk of recurrence is dependent on the number of positive nodes, as well as presence of palpable versus microscopic nodal disease. Further, patients with a metastatic focus of more than 1 mm in greatest dimension in the affected lymph node, have a significantly higher risk of recurrence or death than those with a metastasis of 1 mm or less. Patients with Stage IIIA disease have a disease-specific survival rate of 78% whereas those patients with Stage IIIB and Stage IIIC disease have disease-specific survival rates of 59% and 40% respectively. Several agents are presently approved by the FDA for the adjuvant treatment of high-risk Melanoma and they include YERVOY® (Ipilimumab), OPDIVO® (Nivolumab), TAFINLAR® (Dabrafenib) and MEKINIST® (Trametinib) for BRAF-mutant Melanoma and Interferon alfa. 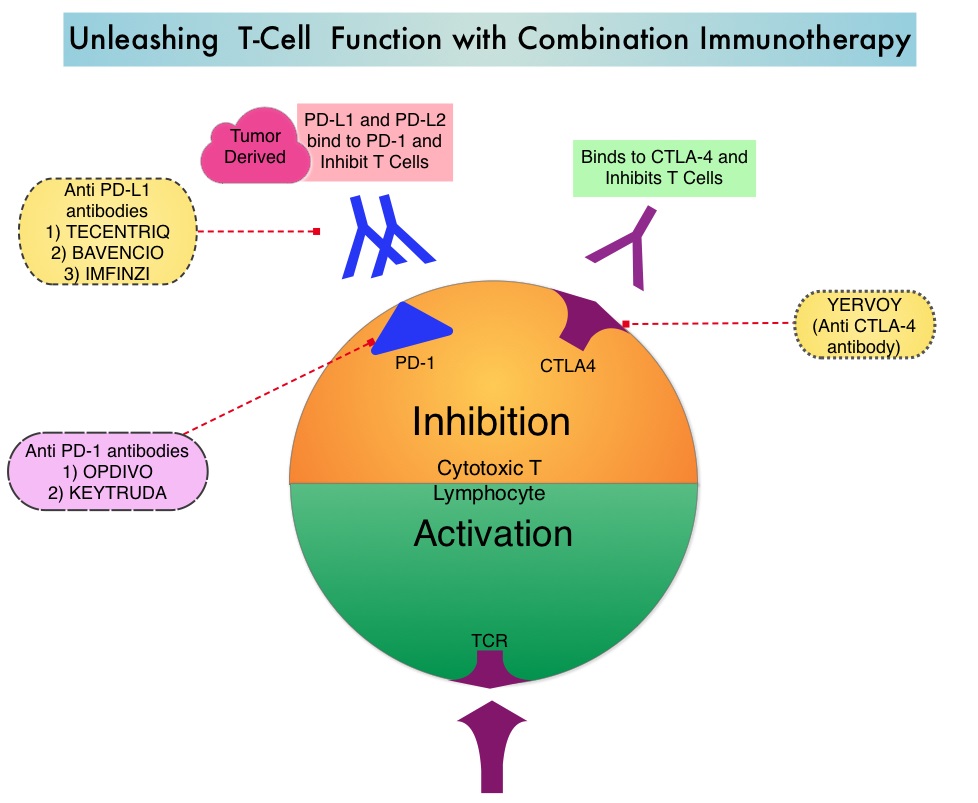
KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. By doing so, it unleashes the tumor-specific effector T cells, and is thereby able to undo PD-1 pathway-mediated inhibition of the immune response.
The present FDA approval was based on the European Organization for Research and Treatment of Cancer (EORTC) 1325/(KEYNOTE-054) trial which is a randomized, double-blind, placebo-controlled Phase III study which involved high-risk patient population of patients with Stage III Melanoma. This study included 1019 patients with completely resected, Stage IIIA (more than 1 mm lymph node metastasis), IIIB or IIIC Melanoma. Patients were randomly assigned 1:1 to receive KEYTRUDA® 200 mg IV every three weeks (N=514) or placebo (N=505), as adjuvant therapy, for a total of 18 doses (approximately 1 year) or until disease recurrence or unacceptable toxicity. Enrolled patients required complete resection of Melanoma with negative margins and lymph node dissection. Patients with mucosal or ocular Melanoma were excluded. The Primary end points were Recurrence-Free Survival (RFS) in the overall intention-to-treat population and in the subgroup of patients with cancer that was positive for the PD-L1, as well as Safety.
At a median follow up of 15 months, KEYTRUDA® was associated with significantly longer Recurrence-Free Survival (RFS) compared to placebo in the overall intent-to-treat population, with a 1-year RFS rate of 75.4% versus 61.0% respectively (HR for recurrence or death=0.57; P<0.001). This suggested that the risk of recurrence or death in the total population was 43% lower in the KEYTRUDA® group than in the placebo group. Patients receiving KEYTRUDA® experienced fewer recurrences/deaths, 26% compared with 43% in the placebo group. The RFS benefit with KEYTRUDA® compared with placebo was observed regardless of tumor PD-L1 expression. In the subgroup of 853 patients with PD-L1-positive tumors, the 1-year RFS rate was 77.1% in the KEYTRUDA® group and 62.6% in the placebo group (HR=0.54; P<0.001). This suggested that the risk was 46% lower in the KEYTRUDA® group than in the placebo group, among patients with PD-L1-positive tumors. KEYTRUDA® was also consistently effective in patients with PD-L1-negative tumors and in those with undetermined tumor PD-L1 expression The Median RFS was 20.4 months in the placebo arm and not reached for those receiving KEYTRUDA®. The most common adverse reactions were rash, asthenia, influenza-like illness, diarrhea, pruritus, nausea, arthralgia and hypothyroidism.
It was concluded that KEYTRUDA® as adjuvant therapy for high-risk Stage III Melanoma, resulted in significantly longer Recurrence-Free Survival than placebo, with no new toxic effects identified. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. Eggermont AM, Blank CU, Mandala M, et al. N Engl J Med 2018;378:1789-1801

