SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. It binds to the extracellular domain of the receptor and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Despite this benefit, majority of these patients develop progressive disease within 18 months. The tumors in these patients continue to express HER2 although the lower sensitivity to HER2 targeted agents has been attributed to HER2 independent escape mechanisms. Treatment strategies for this patient population have included switching chemotherapy in subsequent lines of treatment and continuing HERCEPTIN®, combining another HER2 targeted agent, Lapatinib (TYKERB®) with Capecitabine (XELODA®) and dual HER2 inhibition with a combination of HERCEPTIN® and TYKERB®. KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1) is an antibody-drug conjugate (ADC) comprised of the antibody HERCEPTIN® and the chemotherapy agent Emtansine, linked together. Upon binding to the HER2 receptor, it not only inhibits the HER2 signaling pathways but also delivers a chemotherapy agent Emtansine, a microtubule inhibitor, directly inside the tumor cells. This agent is internalized by lysosomes and destroys the HER2-positive tumor cells upon intracellular release.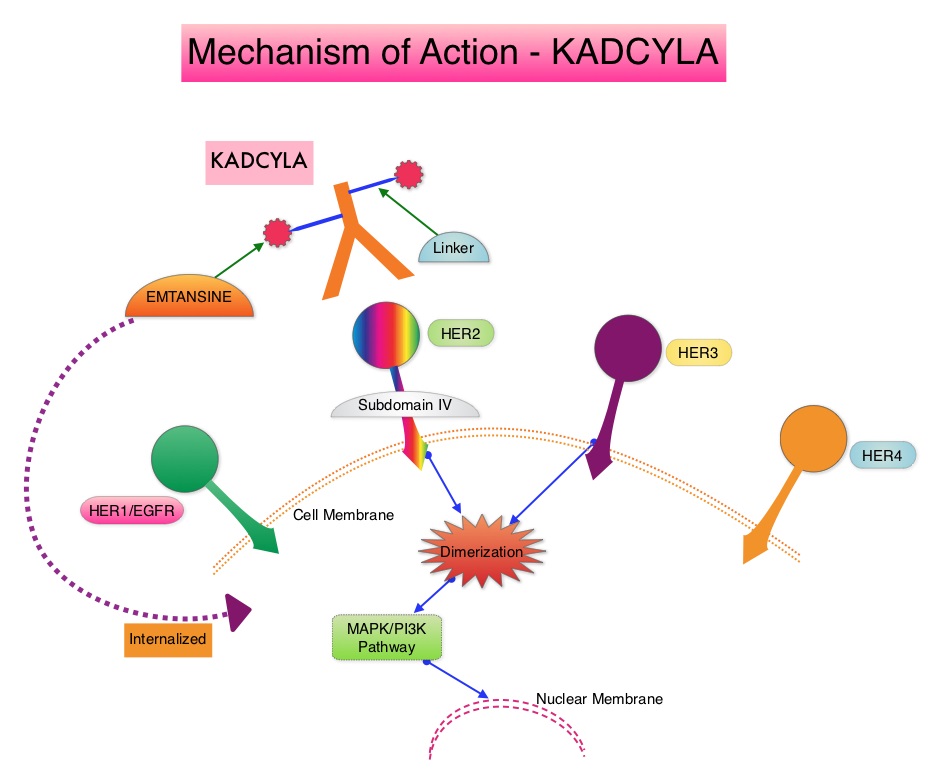 In the EMILIA trial, KADCYLA® was associated with significant increase in Overall Survival when compared with TYKERB® and XELODA® in HER2-positive metastatic breast cancer patients, who had previously received HERCEPTIN® and a taxane. This study however excluded patients who had previously received TYKERB®. TH3RESA is an open label randomized phase III trial in which KADCYLA® was compared with treatment of physician’s choice, in patients with unresectable locally advanced, recurrent or metastatic breast cancer. Eligible patients had a left ventricular ejection fraction of 50% or more, ECOG performance status of 0-2 and had HER2-positive advanced breast cancer who had received two or more HER2-directed regimens in the advanced setting and had progressed on both HERCEPTIN® and TYKERB® containing regimens in metastatic setting and also had disease progression on a taxane, in any setting. Patients were randomized in a 2:1 ratio to receive either KADCYLA® 3•6 mg/kg intravenously every 21 days (N=404) or treatment of physician’s choice (N=198). Treatment was continued until disease progression or unmanageable toxicity. The Co-primary endpoints were Progression Free Survival (PFS) and Overall Survival. Secondary endpoints included Response Rates, duration of response, safety and quality of life. After a median follow up of 7•2 months in the KADCYLA® group and 6•5 months in the physician's treatment choice group, there was a significant improvement in Progression Free Survival with KADCYLA® compared with physician's treatment choice (6•2 months vs 3•3 months, HR= 0•528, P<0•0001). The interim Overall Survival analysis showed a trend favoring KADCYLA® (HR=0•552, P=0•0034). Patients in the KADCYLA® group had a lower incidence of grade 3 toxicities compared to the patients in the physician’s treatment choice group (32% vs 43%). Grade 3 thrombocytopenia however was more common in the KADCYLA® group compared to the physician’s choice group (5% vs 2%) and this has been attributed to the inhibition of megakaryocyte differentiation by KADCYLA®. The authors concluded that KADCYLA® should be considered the treatment of choice, for patients with HER2-positive advanced breast cancer, who have previously received HERCEPTIN® and TYKERB®. It remains to be seen however, if KADCYLA® is effective in patients who had progressed on Pertuzumab (PERJETA®) based therapies. Krop IE, Kim SB, González-Martín A, et al. Lancet Oncol. 2014;15:689-699
In the EMILIA trial, KADCYLA® was associated with significant increase in Overall Survival when compared with TYKERB® and XELODA® in HER2-positive metastatic breast cancer patients, who had previously received HERCEPTIN® and a taxane. This study however excluded patients who had previously received TYKERB®. TH3RESA is an open label randomized phase III trial in which KADCYLA® was compared with treatment of physician’s choice, in patients with unresectable locally advanced, recurrent or metastatic breast cancer. Eligible patients had a left ventricular ejection fraction of 50% or more, ECOG performance status of 0-2 and had HER2-positive advanced breast cancer who had received two or more HER2-directed regimens in the advanced setting and had progressed on both HERCEPTIN® and TYKERB® containing regimens in metastatic setting and also had disease progression on a taxane, in any setting. Patients were randomized in a 2:1 ratio to receive either KADCYLA® 3•6 mg/kg intravenously every 21 days (N=404) or treatment of physician’s choice (N=198). Treatment was continued until disease progression or unmanageable toxicity. The Co-primary endpoints were Progression Free Survival (PFS) and Overall Survival. Secondary endpoints included Response Rates, duration of response, safety and quality of life. After a median follow up of 7•2 months in the KADCYLA® group and 6•5 months in the physician's treatment choice group, there was a significant improvement in Progression Free Survival with KADCYLA® compared with physician's treatment choice (6•2 months vs 3•3 months, HR= 0•528, P<0•0001). The interim Overall Survival analysis showed a trend favoring KADCYLA® (HR=0•552, P=0•0034). Patients in the KADCYLA® group had a lower incidence of grade 3 toxicities compared to the patients in the physician’s treatment choice group (32% vs 43%). Grade 3 thrombocytopenia however was more common in the KADCYLA® group compared to the physician’s choice group (5% vs 2%) and this has been attributed to the inhibition of megakaryocyte differentiation by KADCYLA®. The authors concluded that KADCYLA® should be considered the treatment of choice, for patients with HER2-positive advanced breast cancer, who have previously received HERCEPTIN® and TYKERB®. It remains to be seen however, if KADCYLA® is effective in patients who had progressed on Pertuzumab (PERJETA®) based therapies. Krop IE, Kim SB, González-Martín A, et al. Lancet Oncol. 2014;15:689-699

