The FDA on May 18, 2016 granted accelerated approval to TECENTRIQ® injection for the treatment of patients with locally advanced or metastatic Urothelial carcinoma, who have disease progression during or following Platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with Platinum-containing chemotherapy. TECENTRIQ® is a Programmed Death-ligand 1 (PD-L1) blocking antibody and is a product of Genentech, Inc.
Tag: Urothelial Cancer (Bladder-Ureters-Renal-Pelvis)
FDA Approves TECENTRIQ® for Advanced Urothelial Carcinoma
SUMMARY: The FDA on May 18, 2016 approved TECENTRIQ® (Atezolizumab) for the treatment of patients with locally advanced or metastatic urothelial carcinoma, whose disease has worsened during or following platinum containing chemotherapy, or within 12 months of receiving platinum containing chemotherapy, either before (neoadjuvant) or after (adjuvant) surgical treatment. Urothelial carcinoma accounts for 90 percent of all bladder cancers and can originate in the renal pelvis, ureter and urethra. The National Cancer Institute estimates that in 2016, approximately 76,960 new cases of Bladder Cancer will be diagnosed and 16,390 patients will die of the disease. Treatment options for patients who progress after platinum based chemotherapy are limited, with poor outcomes. The treatment paradigm for solid tumors has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers inhibit an intense immune response by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1) that are expressed by cells in the tumor micro environment. By doing so, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.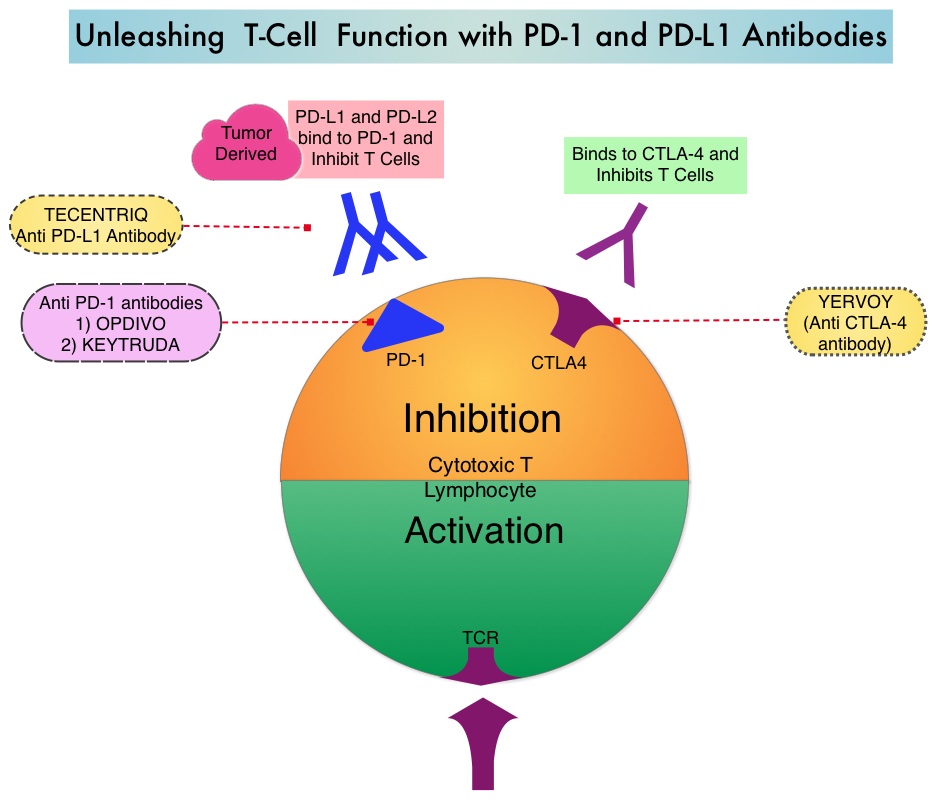
TECENTRIQ® (Atezolizumab) is an anti-PDL1 monoclonal antibody designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating Immune Cells, thereby blocking its interactions with PD-1 and B7.1 receptors and thus enabling the activation of T cells. The FDA approval was based on IMvigor 210 trial which is an open label, single arm, multicenter, phase II study in which the safety and efficacy of TECENTRIQ® was evaluated in patients with locally advanced or metastatic urothelial carcinoma, regardless of PD-L1 expression. In this study, 310 patients whose disease had progressed during or following previous treatment with a platinum based chemotherapy regimen received TECENTRIQ® 1200 mg as an intravenous dose once every 21 days cycles until disease progression. The primary endpoint of the study was Objective Response Rate (ORR) and secondary endpoints included Duration of Response (DOR), Overall Survival (OS), Progression Free Survival (PFS) and safety. PD-L1 expression on tumor-infiltrating Immune Cells (IC) was assessed prospectively by ImmunoHistoChemistry using Formalin-Fixed Paraffin-Embedded tumor specimens. PD-L1 positivity was defined as follows – IC 2/3 (5% or more), IC 1 (1-5%) and IC 0 (<1%).
In an updated analysis, with a median follow of 11.7 months, the ORR was 15% for the entire cohort of patients (P=0.0058). The ORR in the IC 2/3 group was 26% (P<0.0001) and 18% in the IC 1 group (P=0.0004). Ongoing responses were noted in 84% of the responding patients. The most common side effects associated with TECENTRIQ® included fatigue, decreased appetite, nausea, urinary tract infection, fever and constipation.
The authors concluded that TECENTRIQ® significantly improves Objective Response rate (ORR) in metastatic urothelial cancers and this benefit is even more so in those tumors with a higher level of PD-L1 expression. Rosenberg JE, Hoffman-Censits, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; Published online 4 March. DOI: http://dx.doi.org/10.1016/S0140-6736(16)00561-4
Clinical Guidelines for the Evaluation of Hematuria as a Marker of Occult Urinary Tract Cancer
SUMMARY: Hematuria is a common finding in clinical practice and millions of patients have routine urine evaluation. However, the magnitude of the risk for an underlying malignancy can vary significantly. Screening healthy, asymptomatic patients with urinalysis for malignancy is not currently recommended by any major health organization. This article describes the indications for the evaluation of hematuria, as a marker of occult urinary tract cancer, and is meant to help Health Care Providers make appropriate referral of patients, for urologic evaluation. The information in this article was gathered following a review of published clinical guidelines that addressed the evaluation of hematuria as a marker of occult urinary tract cancer, as well as other relevant studies, from the peer-reviewed literature. The American College of Physicians' High Value Care Task Force put this information together, with the intent to increase awareness and provide practical advice, based on the best available evidence.
High-Value Care Advice 1: Clinicians should include gross hematuria in their routine review of systems and specifically ask all patients with microscopic hematuria about any history of gross hematuria.
High-Value Care Advice 2: Clinicians should not use screening urinalysis for cancer detection in asymptomatic adults.
High-Value Care Advice 3: Clinicians should confirm heme-positive results of dipstick testing with microscopic urinalysis that demonstrates 3 or more erythrocytes per high-powered field before initiating further evaluation in all asymptomatic adults.
High-Value Care Advice 4: Clinicians should refer all adults with gross hematuria, even if self-limited, for further urologic evaluation.
High-Value Care Advice 5: Clinicians should consider urology referral for cystoscopy and imaging in adults with microscopically confirmed hematuria, in the absence of some demonstrable benign cause.
High-Value Care Advice 6: Clinicians should pursue evaluation of hematuria even if the patient is receiving antiplatelet or anticoagulant therapy.
High-Value Care Advice 7: Clinicians should not obtain urinary cytology or other urine-based molecular markers for bladder cancer detection in the initial evaluation of hematuria.
Hematuria as a Marker of Occult Urinary Tract Cancer: Advice for High-Value Care from the American College of Physicians. Nielsen M, Qaseem A, for the High Value Care Task Force of the American College of Physicians. Ann Intern Med. 2016;164:488-497.
Molecular Markers Can Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle Invasive Bladder Cancer
SUMMARY: The American Cancer Society estimates that in the United States for 2015, about 74,000 new cases of bladder cancer will be diagnosed and approximately 16,000 patients will die of the disease. A third of the patients initially present with locally invasive or metastatic disease. Even though radical cystectomy was considered the standard of care for patients with localized Muscle Invasive Bladder Cancer (MIBC), two large randomized trials and two meta-analysis have shown greater survival benefit with neoadjuvant Cisplatin-based chemotherapy combinations for patients with MIBC, compared to surgery alone. However, not all patients with MIBC benefit from neoadjuvant Cisplatin based therapy, with only 25-50% attaining a pathologic response. The authors in this publication evaluated the role of genetic testing and genetic biomarkers prior to chemotherapy, in order to select the appropriate group of patients who would benefit from neoadjuvant Cisplatin based chemotherapy and thereby exclude those who are unlikely to benefit from chemotherapy.
Muscle Invasive Bladder Cancer (MIBC) patients from two prospective multicenter clinical trials of Cisplatin-based neoadjuvant chemotherapy were included and pretreatment MIBC samples were prospectively collected and this provided the discovery and validation sets. DNA from pre-treatment tumor tissue was sequenced for all coding exons of 287 cancer related genes and was analyzed for presence of base substitutions, indels, copy number alterations and selected rearrangements. The discovery set allowed for improved prognostic strength, by combining existing known high risk genetic markers with other novel risk markers. The validation set validated these markers. This study included data from 34 patients in the discovery cohort and 24 patients in the validation cohort.
It was noted that patients with a pathologic Complete Response had more alterations in the genes compared to those with residual tumor, both in the discovery (P= 0.024) and validation (P=0.018) sets. Further, in the discovery set, alteration in one or more of the three DNA repair genes ATM, RB1, and FANCC predicted pathologic Complete Response (P<0.001; 87% sensitivity, 100% specificity) as well as improved Overall Survival (P=0.007). DNA repair is one of the mechanisms postulated to contribute to Cisplatin chemo-resistance. This test remained predictive for pathologic Complete Response in the validation set (P=0.033), with a trend towards improved Overall Survival (P=0.055).
The authors concluded that defective DNA repair renders tumors sensitive to Cisplatin and genomic alterations in the DNA repair-associated genes ATM, RB1, and FANCC predict response and clinical benefit for MIBC, treated with Cisplatin-based chemotherapy. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Plimacka ER, Dunbracka RL, Brennan TA, et al. European Urology 2015;68:959-967
Vitamin D deficiency and Bladder Cancer
The active metabolite of Vitamin D is Vitamin D3. In a recent study published in the JNCI, September 2012 issue, the authors noted a statistically significant increase in the risk of Bladder Cancer in individuals with low plasma concentrations of Vitamin D3. It is felt that Vitamin D3 favorably upregulates Fibroblast Growth Factor Receptor 3 (FGFR3). Mutations in FGFR3 are known to be associated with urothelial carcinoma. Urine samples evaluating for FGFR3 mutations, to diagnose urothelial carcinoma, has a positive predictive value of 95% when detected in patients with no history of Bladder Cancer. It appears that patients with aggressive bladder cancer have low expression of wild- type FGFR3 in association with low plasma concentrations of Vitamin D3. Therefore, it is possible that low plasma concentrations of Vitamin D3 may predict increased risk for bladder cancer, with more aggressive types of bladder cancer manifesting in those with significantly low Vitamin D3 levels.
Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer
SUMMARY: In this phase III trial, 360 patients with muscle-invasive bladder cancer were randomized to receive radiation therapy with or without concurrent chemotherapy. The chemotherapy regimen consisted of 5-fluorouracil given on days 1-5 and days16-20 of radiotherapy and mitomycin C given on day 1. The primary end point of this study was locoregional disease free survival. Secondary end points included overall survival and toxicities. The locoregional disease–free survival at two years was 67% in the chemoradiation group and 54% in the radiation therapy only group. With a median follow-up of 69.9 months, the hazard ratio in the chemoradiation group was 0.68 (P=0.03). The overall survival at five years was 48% in the chemoradiation group and 35% in the radiation therapy only group with a hazard ratio of 0.82 (P=0.16). The authors concluded that for patients with muscle invasive bladder cancer, concurrent chemoradiation significantly improved locoregional disease free survival compared to radiation therapy only. James ND, Hussain SA, Hall E, et al. N Engl J Med 2012; 366:1477-1488
