SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 8 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 248,530 new cases of prostate cancer will be diagnosed in 2021 and 34,130 men will die of the disease.
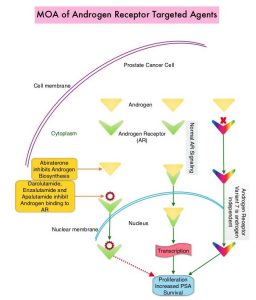
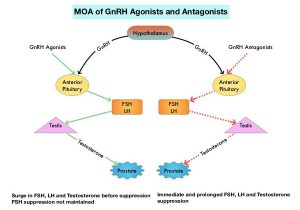
The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) or testosterone suppression has therefore been the cornerstone of treatment of advanced prostate cancer, and is the first treatment intervention. Approximately 10-20% of patients with advanced Prostate cancer will progress to Castration Resistant Prostate Cancer (CRPC) within five years during ADT, and over 80% of these patients will have metastatic disease at the time of CRPC diagnosis. Among those patients without metastases at CRPC diagnosis, 33% are likely to develop metastases within two years. Progression to Castration Resistant Prostate Cancer (CRPC) often manifests itself with a rising PSA (Prostate Specific Antigen) and the estimated mean survival of patients with CRPC is 9-36 months, and there is therefore an unmet need for new effective therapies.
Prostate-Specific Membrane Antigen (PSMA) is a type II cell membrane glycoprotein that is selectively expressed in prostate cells, with high levels of expression in prostatic adenocarcinoma. PSMA is a therefore an excellent target for molecular imaging and therapeutics, due to its high specificity for prostate cancer.
Lu-177-PSMA-617 is a radiopharmaceutical that targets PSMA. It is comprised of Lutetium-177, linked to the ligand PSMA-617, a small molecule designed to bind with high affinity to PSMA. Radioligand therapy with Lu-177-PSMA-617 targets PSMA and releases its payload of lethal beta radiation into the prostate cancer cell. The antitumor activity and safety of Lu-177-PSMA-617 have been established previously in a Phase II study (Lancet Oncol. 2018;19:825-833).
VISION is an international, randomized, open-label Phase III study in which the benefit of Lu-177-PSMA-617 was evaluated in men with PSMA-positive mCRPC, previously treated with second generation Androgen Receptor signaling pathway inhibitor (XTANDI®-Enzalutamide or ZYTIGA®-Abiraterone acetate), and 1 or 2 taxane chemotherapy regimens. In this trial, 831 patients were randomized 2:1 to receive Lu-177-PSMA-617, 7.4 GBq every 6 weeks for 6 cycles plus Standard of Care as determined by the treating physician (N=551), or Standard of Care only (N=280). Both treatment groups were well balanced and this trial excluded patients treated with XOFIGO® (Radium-223). Enrolled patients had a castrate level or serum/plasma testosterone of lower than 50 ng/dL, and PET imaging with 68 Ga-PSMA-11 was used to determine PSMA positivity by central review. The Primary endpoints were radiographic Progression Free Survival (rPFS) by Independent Central Review (ICR) and Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Disease Control Rate (DCR), and time to first Symptomatic Skeletal Event (SSE). The median study follow up was 20.9 months.
Lu-177-PSMA-617 plus Standard of Care significantly improved rPFS by 60%, compared to Standard of Care alone (median rPFS 8.7 versus 3.4 months, HR=0.40; P<0.001). The median OS was also significantly improved by 38% with Lu-177-PSMA-617 plus Standard of Care compared to Standard of Care alone (median OS 15.3 versus 11.3 months, HR=0.62; P<0.001). All key secondary endpoints including Objective Response Rate, Disease Control Rate, and time to first Symptomatic Skeletal Event were statistically significant, and in favor of Lu-177-PSMA-617 plus Standard of Care.
It was concluded that radioligand therapy with Lutetium-177–PSMA-617 significantly improved radiographic Progression Free Survival and Overall Survival when added to Standard of Care, compared with Standard of Care alone, in men with PSMA-positive metastatic Castration Resistant Prostate Cancer.
Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. Sartor O, de Bono J, Chi KN, et al. N Engl J Med 2021; 385:1091-1103.