SUMMARY: The American Cancer Society estimates that in 2017, about 53,670 people will be diagnosed with pancreatic cancer in the United States and about 43,090 patients will die of the disease. Some important risk factors for pancreatic cancer include increasing age, obesity, smoking history, genetic predisposition, exposure to certain dyes and chemicals, heavy alcohol use and pancreatitis. The best chance for long term survival is complete surgical resection, although this may not be feasible in a majority of the patients, as they present with advanced disease at the time of diagnosis. Based on the National Cancer Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the pancreas is 14% for those with Stage IA disease and 1% for those with Stage IV disease. The FDA approved ABRAXANE® ((Paclitaxel albumin-bound particles) for use, in combination with GEMZAR® (Gemcitabine), for the first line treatment of patients with metastatic adenocarcinoma of the pancreas. This approval was based on the demonstration of improved Overall Survival (OS) and Progression Free Survival (PFS) with this combination, when compared to single agent GEMZAR®, in a multicenter, international, open-label, randomized trial (MPACT study).
PEGPH20 is a PEGylated form of recombinant human Hyaluronidase, for the potential systemic treatment of tumors that accumulate Hyaluronan (HA). PEGPH20 is an enzyme that temporarily degrades Hyaluronan, a dense component of the tumor microenvironment that can accumulate in higher concentrations around certain cancer cells and potentially constrict blood vessels and there by impede treatment access to tumor tissue. It is estimated that 35% to 40% of patients with pancreatic cancer have high expression of Hyaluronan and this biomarker may predict response to PEGPH20.
HALO-202 is a phase 2 multicenter, randomized clinical trial, in which PEGPH20 in combination with ABRAXANE® and GEMZAR® (N=166) – PAG, was compared with ABRAXANE® and GEMZAR® – AG (N=113), in treatment-naive patients, with metastatic pancreatic carcinoma. In this study, following enrollment of 146 patients in the first stage of the trial, the study was placed on hold to address concerns regarding thromboembolic events, in the group receiving PEGPH20. The protocol was amended to exclude those at high risk for a thromboembolic event and prophylaxis with Low Molecular Weight Heparin was required. One hundred thirty-three patients (N=133) were enrolled into the second stage of the trial for a total of 279 patients. Patients enrolled in stage 2 received Low Molecular Weight Heparin at a starting dose of 40 mg/day or 1 mg/kg/day, to prevent thromboembolic events. Each 4-week treatment cycle consisted of 3 weekly treatments and 1 week off. PEGPH20 was administered at 3 µg/kg twice weekly for cycle 1 followed by weekly administration in subsequent cycles. ABRAXANE® and GEMZAR® were administered weekly at their standard FDA-approved doses of 125 mg/m2 and 1,000 mg mg/m2 respectively. Tumor biopsy samples for the Hyaluronan analysis were available for 138 patients treated with PEGPH20 and 79 patients treated in the control group, across both stages of the study. Overall, 49 patients in the PEGPH20 arm and 35 in the control group had Hyaluronan expression of 50% or more. The Primary endpoint of the study was Progression Free Survival (PFS) across the entire treatment group. Following change in the treatment protocol, a second Primary endpoint was added to assess thromboembolic event rate. Secondary endpoints included Objective Response Rate, PFS by Hyaluronan level, and Overall Survival. The second stage of this study was also utilized to validate a companion diagnostic for Hyaluronan (HA) levels.
It was noted that across the overall study population, there was a statistically significant increase in Progression Free Survival (PFS) in the PEGPH20 group compared to the control group (6 months versus 5.2 months; HR=0.73; P=0.49). In patients with high levels of Hyaluronan (HA-High), the PFS was 9.2 months among those treated with PEGPH20 plus ABRAXANE® and GEMZAR® versus 5.2 months among patients receiving ABRAXANE® and GEMZAR® alone (HR = 0.51, P = 0.048). The additional Primary endpoint of a reduction in the rate of thromboembolic events was achieved, in the PEGPH20 group. Across all patients, thromboembolic events were experienced by 14% of those in the PEGPH20 group versus 10% of those in the ABRAXANE® and GEMZAR® group. These events were lower in those receiving Low Molecular Weight Heparin at 1 mg/kg/day dose versus 40 mg/day (6% vs 10%, respectively). The most common adverse events were cytopenias.
The authors concluded that the addition of PEGPH20 to ABRAXANE® and GEMZAR® resulted in significant improvement in Progression Free Survival compared with ABRAXANE® plus GEMZAR® alone, in treatment naïve patients with advanced pancreatic cancer. Patients with high levels of expression of the biomarker Hyaluronan, had the best outcomes suggesting that a biopsy-based biomarker for hyaluronan content can potentially identify patients who will have a meaningfully greater response when PEGPH20 is added to standard chemotherapy. A phase III study is underway, evaluating PEGPH20 in combination with ABRAXANE® and GEMZAR® in patients with metastatic pancreatic cancer, with high Hyaluronan levels. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. Hingorani SR, Zheng L, Bullock AJ, et al. DOI: 10.1200/JCO.2017.74.9564 Journal of Clinical Oncology – published online before print December 12, 2017

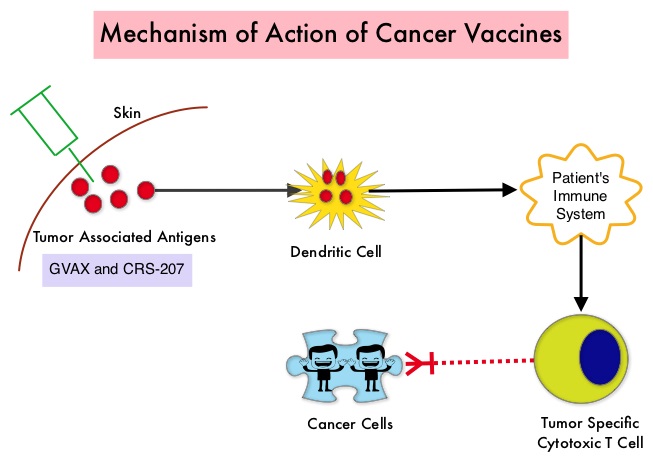 The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
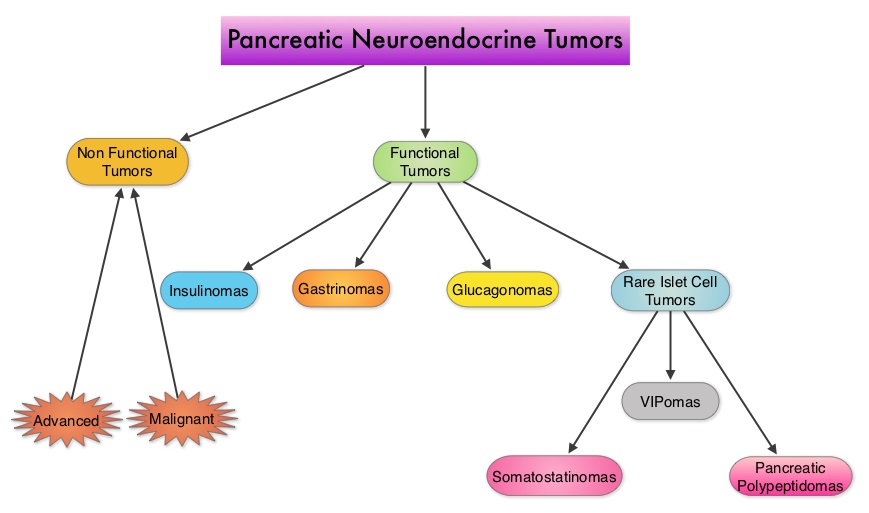 They account for approximately 25% of all neuroendocrine tumors. Pancreatic NETs may be functional or nonfunctional. Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones. Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms. Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer. Predictors of worst survival include advanced stage, higher grade and age. Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor. RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care. The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR®. Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR®. Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR®. The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group (P < 0.001). The authors now report the mature Overall Survival results. The median Overall Survival was 44 months in the AFINITOR® group compared with 37.7 months in the placebo arm. The 6.3 month survival difference in favor of AFINITOR® was not statistically significant (P= 0.30). The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR®. The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue. The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors. Yao J, Pavel M, Lombard-Bohas C, et al. ESMO 2014 Congress, Abstract#11320
They account for approximately 25% of all neuroendocrine tumors. Pancreatic NETs may be functional or nonfunctional. Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones. Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms. Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer. Predictors of worst survival include advanced stage, higher grade and age. Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor. RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care. The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR®. Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR®. Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR®. The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group (P < 0.001). The authors now report the mature Overall Survival results. The median Overall Survival was 44 months in the AFINITOR® group compared with 37.7 months in the placebo arm. The 6.3 month survival difference in favor of AFINITOR® was not statistically significant (P= 0.30). The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR®. The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue. The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors. Yao J, Pavel M, Lombard-Bohas C, et al. ESMO 2014 Congress, Abstract#11320