SUMMARY: The American Cancer Society estimates that in 2014, over 46,000 people will be diagnosed with Pancreatic Cancer in the United States and close to 40,000 people will die of the disease. Some important risk factors for Pancreatic Cancer include increasing age, obesity, smoking history, genetic predisposition, exposure to certain dyes and chemicals, heavy alcohol use and pancreatitis. The best chance for long term survival is complete surgical resection, although this may not be feasible in a majority of the patients, as they present with advanced disease at the time of diagnosis. Based on the National Cancer Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the pancreas is 14% for those with Stage IA disease and 1% for those with Stage IV disease. The FDA recently granted Breakthrough Therapy Designation status for the combination treatment that consists of two vaccines, GVAX and CRS-207, for patients with advanced Pancreatic Carcinoma. This designation was based on the following study. A phase II clinical trial was conducted in which the authors took a novel approach and tested a combination of two vaccines in patients with metastatic pancreatic adenocarcinoma.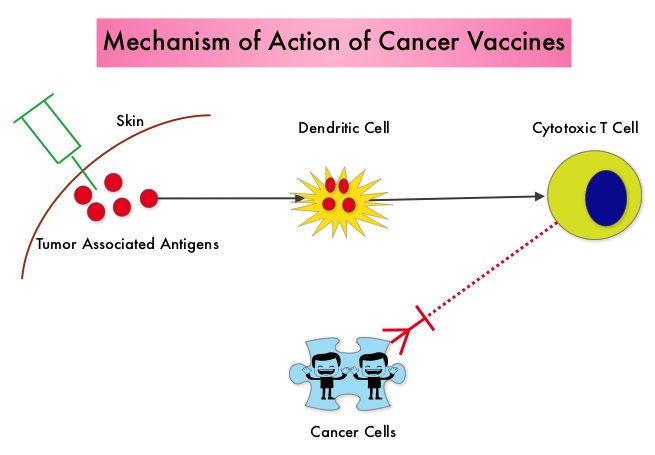 Traditional vaccination against specific bacterial and viral infections involves the injection of the specific weakened bacteria/virus or a structural component of the bacteria or virus. The body then mounts an immune response and is ready to respond to an infection associated with that specific bacteria or virus. Use of vaccines in cancer treatment is based on the same principle. The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from pancreatic cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on pancreatic cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic pancreatic adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 or six doses of GVAX alone. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median overall survival was 6.1 months with the combination of two vaccines vs 3.9 months with GVAX alone (HR=0.54, P=0.011), a 46% reduction in risk of death with the combination immunotherapy. The median overall survival in patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for GVAX alone (HR=0.44, P=0.0074), a 56% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median overall survival of 5.1 months vs 3.7 months with GVAX alone (HR=0.34, P=0.001), a 66% reduction in risk of death. Stabilization of tumor marker CA19-9, was seen in 32% of patients receiving combination immunotherapy vs 13% in those who received GVAX alone (P=0.06). The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group and 12% for the GVAX alone group. Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved overall survival in patients with metastatic pancreatic carcinoma, who had failed prior therapies. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 32, 2014 (suppl 3; abstr 177)
Traditional vaccination against specific bacterial and viral infections involves the injection of the specific weakened bacteria/virus or a structural component of the bacteria or virus. The body then mounts an immune response and is ready to respond to an infection associated with that specific bacteria or virus. Use of vaccines in cancer treatment is based on the same principle. The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from pancreatic cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on pancreatic cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic pancreatic adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 or six doses of GVAX alone. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median overall survival was 6.1 months with the combination of two vaccines vs 3.9 months with GVAX alone (HR=0.54, P=0.011), a 46% reduction in risk of death with the combination immunotherapy. The median overall survival in patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for GVAX alone (HR=0.44, P=0.0074), a 56% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median overall survival of 5.1 months vs 3.7 months with GVAX alone (HR=0.34, P=0.001), a 66% reduction in risk of death. Stabilization of tumor marker CA19-9, was seen in 32% of patients receiving combination immunotherapy vs 13% in those who received GVAX alone (P=0.06). The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group and 12% for the GVAX alone group. Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved overall survival in patients with metastatic pancreatic carcinoma, who had failed prior therapies. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 32, 2014 (suppl 3; abstr 177)
Tag: Pancreatic Cancer
Increased Risk of Pancreatic Adenocarcinoma After Acute Pancreatitis
SUMMARY: The American Cancer Society estimates that in 2014, over 46,000 people will be diagnosed with Pancreatic Cancer in the United States and close to 40,000 people will die of the disease. Some important risk factors for Pancreatic Cancer include increasing age, obesity, smoking history, genetic predisposition, exposure to certain dyes and chemicals, heavy alcohol use and pancreatitis. The best chance for long term survival is complete surgical resection, although this may not be feasible in a majority of the patients, as they present with advanced disease, at the time of diagnosis. Based on the National Cancer Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the pancreas is 14% for those with Stage IA disease and 1% for those with Stage IV disease. Early diagnosis may therefore play an important role in treatment outcomes, in patients with Pancreatic Cancer. Pancreatic Cancer can cause acute pancreatitis by obstructing the pancreatic duct and patients diagnosed with Pancreatic Cancer often present initially with acute pancreatitis. With this background information, the authors performed a retrospective study of patients with acute pancreatitis who sought their medical care at the Veterans Health Administration from 1998 through 2007. 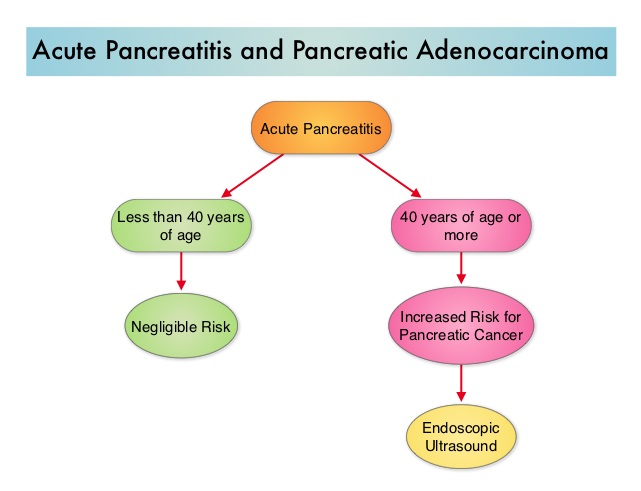 Patients with pre-existing Pancreatic Adenocarcinoma or those with recurrent acute pancreatitis were excluded from this analysis. A diagnosis of acute pancreatitis was made in 5720 patients and 710 patients were diagnosed with Pancreatic Cancer from 2000 through 2007. They noted that of those who were diagnosed with Pancreatic Adenocarcinoma, 76 patients (10.7%) had acute pancreatitis within 2 years of Pancreatic Cancer diagnosis. This risk for Pancreatic Cancer was greatest during the ï¬rst year following diagnosis of acute pancreatitis and this risk decreased rapidly thereafter. Patients less than 40 years of age had negligible risk whereas those 70 years of age or older had the highest risk. The authors concluded that a signiï¬cant number of patients with Pancreatic Adenocarcinoma (12.1%) initially present with acute pancreatitis and the diagnosis of cancer is often delayed by up to 2 years. Acute pancreatitis should be considered as an index event which in turn may identify a population of patients at high risk to develop Pancreatic Adenocarcinoma. They recommend that Endoscopic UltraSound (EUS) should be performed in these high risk patients, following diagnosis of acute pancreatitis, before discharge from the hospital, as this test is highly sensitive in picking up small tumors in the pancreas that are amenable to surgical resection. This is in comparison with contrast enhanced CT scans and pancreatic protocol CT scans that are not as sensitive in identifying tumors less than 2 cm in size. This interesting analysis could potentially open the doors for pancreatic cancer screening in high risk patients. Munigala S, Kanwal F, Xian H, et al. Clinical Gastroenterology and Hepatology 2014;12:1143-1150
Patients with pre-existing Pancreatic Adenocarcinoma or those with recurrent acute pancreatitis were excluded from this analysis. A diagnosis of acute pancreatitis was made in 5720 patients and 710 patients were diagnosed with Pancreatic Cancer from 2000 through 2007. They noted that of those who were diagnosed with Pancreatic Adenocarcinoma, 76 patients (10.7%) had acute pancreatitis within 2 years of Pancreatic Cancer diagnosis. This risk for Pancreatic Cancer was greatest during the ï¬rst year following diagnosis of acute pancreatitis and this risk decreased rapidly thereafter. Patients less than 40 years of age had negligible risk whereas those 70 years of age or older had the highest risk. The authors concluded that a signiï¬cant number of patients with Pancreatic Adenocarcinoma (12.1%) initially present with acute pancreatitis and the diagnosis of cancer is often delayed by up to 2 years. Acute pancreatitis should be considered as an index event which in turn may identify a population of patients at high risk to develop Pancreatic Adenocarcinoma. They recommend that Endoscopic UltraSound (EUS) should be performed in these high risk patients, following diagnosis of acute pancreatitis, before discharge from the hospital, as this test is highly sensitive in picking up small tumors in the pancreas that are amenable to surgical resection. This is in comparison with contrast enhanced CT scans and pancreatic protocol CT scans that are not as sensitive in identifying tumors less than 2 cm in size. This interesting analysis could potentially open the doors for pancreatic cancer screening in high risk patients. Munigala S, Kanwal F, Xian H, et al. Clinical Gastroenterology and Hepatology 2014;12:1143-1150
A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma Updated results
SUMMARY: The authors in this study took a novel approach and tested a combination of two vaccines in patients with metastatic pancreatic adenocarcinoma. Traditional vaccination against specific bacterial and viral infections involves the injection of the specific weakened bacteria/virus or a structural component of the bacteria or virus. The body then mounts an immune response and is ready to respond to an infection associated with that specific bacteria or virus. Use of vaccines in cancer treatment is based on the same principle. The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from pancreatic cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on pancreatic cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic pancreatic adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 or six doses of GVAX alone. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median overall survival was 6.1 months with the combination of two vaccines vs 3.9 months with GVAX alone (HR=0.54, P=0.011), a 46% reduction in risk of death with the combination immunotherapy. The median overall survival in patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for GVAX alone (HR=0.44, P=0.0074), a 56% reduction in the risk of death. In the subgroup of patients who had had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median overall survival of 5.1 months vs 3.7 months with GVAX alone (HR=0.34, P=0.001), a 66% reduction in risk of death. Stabilization of tumor marker CA19-9 was seen in 32% of patients receiving combination immunotherapy vs 13% in those who received GVAX alone (P=0.06). The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group and 12% for the GVAX alone group. Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved overall survival in patients with metastatic pancreatic carcinoma, who have failed prior therapies. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 32, 2014 (suppl 3; abstr 177)
Adjuvant Chemotherapy With Gemcitabine and Long-term Outcomes Among Patients With Resected Pancreatic Cancer
SUMMARY: Curative surgical resection has been shown to significantly improve Overall Survival (OS) when compared to Chemoradiation, for resectable Pancreatic Cancer. The standard surgical procedure for tumors of the Pancreatic head is the Pancreaticoduodenectomy (Whipple procedure), whereas distal Pancreatectomy is performed for tumors of the body or tail of the Pancreas. The role of adjuvant chemotherapy following surgery has however remained unclear. In this community based, phase III trial, 368 patients were randomly assigned to receive either adjuvant chemotherapy with GEMZAR® (Gemcitabine) (N=186) or Observation (N=182), following curative resection of the pancreas (Macroscopic complete removal of Pancreatic cancer). Chemotherapy consisted of 6 cycles of GEMZAR® with each cycle consisting of GEMZAR® 1000mg/m2, given weekly, 3 out of 4 weeks. Patients were stratified based on tumor stage (T), nodal status (N) and resection (R) status. The primary endpoint was Disease Free Survival (DFS). Secondary endpoints included OS and safety. With a median follow up of 136 months, the median DFS was 13.4 months in the treatment group vs 6.7 months in the observation group (HR=0.55; P<0.001). The OS in the treatment group was significantly prolonged (HR=0.76; P=0.01), with a 5 year survival of 21% and 10 year survival of 12% compared to 10% and 8% respectively, in the Observation group. The authors concluded that 6 months of GEMZAR® based adjuvant therapy improves Overall Survival for patients with resectable Pancreatic Cancer. There may be added benefit with regimens associated with higher remission rates such as FOLFIRINOX or weekly ABRAXANE® (Paclitaxel albumin-bound particles) and GEMZAR®. Oettle H, Neuhaus P, Hochhaus A, et al. JAMA. 2013;310:1473-1481.
ABRAXANE® TO THE RESCUE
The recent approval by the FDA of ABRAXANE® in combination with GEMZAR® (Gemcitabine) for the first line treatment of patients with metastatic Adenocarcinoma of the Pancreas gives the Oncology Health Care Providers a much needed option to treat this group of patients. With a significant improvement in the Overall Survival, Progression Free Survival and Response Rates, ABRAXANE® combination will very likely replace the more toxic FOLFIRI regimen by virtue of its efficacy and tolerability.
Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT)
SUMMARY: The FDA recently approved ABRAXANE® ((Paclitaxel albumin-bound particles) for use in combination with GEMZAR® (Gemcitabine) for the first line treatment of patients with metastatic adenocarcinoma of the pancreas. This approval was based on the demonstration of improved overall survival (OS) in a multi-center, international, open-label, randomized trial. Eight hundred and sixty one (861) patients with metastatic pancreatic cancer were randomized to receive either the combination of ABRAXANE® and GEMZAR® (n=431) or GEMZAR® alone (n=430). Patients were stratified based on geographic region, performance status, and presence of liver metastasis. The median age was 63 years. The primary end point was OS and secondary endpoints included progression-free survival (PFS) and overall response rate (ORR. There was a statistically significant prolongation of OS for patients in the combination group with a 28% reduction in the risk of death [HR= 0.72; P < 0.0001]. The median OS was 8.5 months in the combination group and 6.7 months in the single agent GEMZAR® group. There was in addition a significant improvement in the PFS in the combination arm vs the single agent arm (5.5 months vs 3.7 months, respectively.HR= 0.69; P < 0.0001). Objective response rates were 23% in the combination group and 7% in the single agent GEMZAR® group (P<0.0001). Serious adverse reactions in patients receiving combination therapy included fever, vomiting, dehydration and pneumonia. This is clearly a major development in the management of advanced pancreatic cancer patients. Von Hoff DD, Ervin TJ, Arena FP, et al. J Clin Oncol 30: 2012 (suppl 34; abstr LBA148)
ABRAXANE® (Paclitaxel albumin-bound particles)
The FDA on September 6, 2013 approved ABRAXANE® for use in combination with GEMZAR® (Gemcitabine) for the first line treatment of patients with metastatic adenocarcinoma of the pancreas. ABRAXANE® is an injectable suspension and is a product of Celgene Corporation.
Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors
SUMMARY: Pancreatic neuroendocrine tumors account for approximately 5% of all pancreatic tumors and in general tend to be indolent tumors. In a randomized multicenter phase III trial, 171 patients with advanced, well-differentiated pancreatic neuroendocrine tumors, whose tumors had progressed in the prior 12 months were randomized to receive either Sunitinib (SUTENT®), a multitargeted tyrosine kinase inhibitor, at a dose of 37.5 mg qd or placebo. Following an interim analysis, this study was closed earlier than planned based on the superiority of SUTENT® over placebo. The median progression free survival was longer for those who received SUTENT® than those in the placebo group (11.4 versus 5.5 months). More patients in the SUTENT® group were alive at 6 months compared to the placebo group (92.6% versus 85.2%). N Engl J Med 2011; 364:501-513
Everolimus for Advanced Pancreatic Neuroendocrine Tumors
SUMMARY: Everolimus (AFINITOR®) is an oral mTOR (mammalian target of rapamycin) inhibitor, presently approved for the treatment of advanced renal cel carcinoma. Based on the established efficacy of AFINITOR® in phase II trials, a prospective, randomized, phase III study was conducted in which 410 patients with advanced, low grade or intermediate grade pancreatic neuroendocrine tumors with progression within the previous 12 months were randomized to receive AFINITOR®, at a dose of 10 mg QD (207 patients), or placebo (203 patients).The primary end point, progression free survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group. These benefits were accomplished with a low rate of grade III and IV toxicities. It appears that the mTOR pathway may play an important role in the molecular pathogenesis of pancreatic neuroendocrine tumors. N Engl J Med 2011; 364:514-523
Randomized phase III trial comparing FOLFIRINOX (F 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA) Preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial
SUMMARY: Adenocarcinoma of the pancreas is one of the hard-to-treat cancers for which chemotherapy has not demonstrated any survival benefit – that is, until now. In a recently presented randomized phase III trial at the ASCO 2010 meeting, 250 patients with metastatic pancreatic cancer were assigned to receive either single agent Gemcitabine (GEMZAR®) or a combination of fluorouracil, leucovorin, Irinotecan (CAMPTOSAR®) , and Oxaliplatin (ELOXATIN®) – (FOLFIRINOX regimen). Following an interim analysis, this trial had to be closed earlier than planned, based on the significantly positive results noted with the combination regimen. The median overall survival for patients in the FOLFIRINOX was 11.1 months compared with 6.8 months for those receiving single agent GEMZAR®. At one year, 48% of patients in the FOLFIRINOX group were alive compared to 20% for those in the GEMZAR® group. The median progression free survival was 6.4 months for the patients treated with FOLFIRINOX compared to 3.3 months for those treated with single agent GEMZAR®. Quality of life was also superior in the FOLFIRINOX group compared to those who were treated with GEMZAR®. For the very first time, we now have a combination chemotherapy regimen for advanced pancreatic cancer that confers survival benefit. J Clin Oncol 28:303s, 2010 (suppl; abstr 4010)
