SUMMARY: The American Cancer Society estimates that for 2020, about 57,600 people will be diagnosed with pancreatic cancer and about 47,050 people will die of the disease. Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and Western Europe. Unfortunately, unlike other malignancies, very little progress has been made and outcome for patients with advanced pancreatic cancer has been dismal, with a 5-year survival rate for metastatic pancreatic cancer of approximately 9%.
Patients with metastatic Pancreatic Ductal AdenoCarcinoma (PDAC) are often treated with chemotherapy and treatment regimens include FOLFIRINOX and Gemcitabine with nab-Paclitaxel (ABRAXANE®). However, resistance to current treatment modalities is common, and the median Overall Survival (OS) remains less than 1 year, suggesting that treatment with chemotherapy alone probably may not increase response rates and Overall Survival.
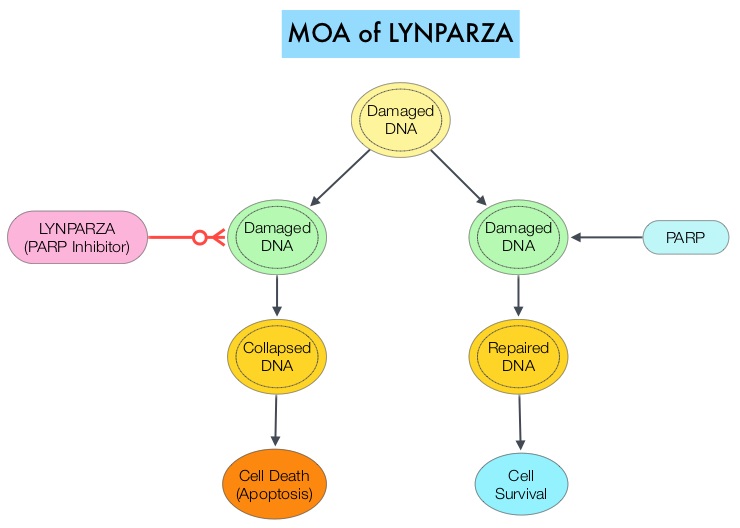
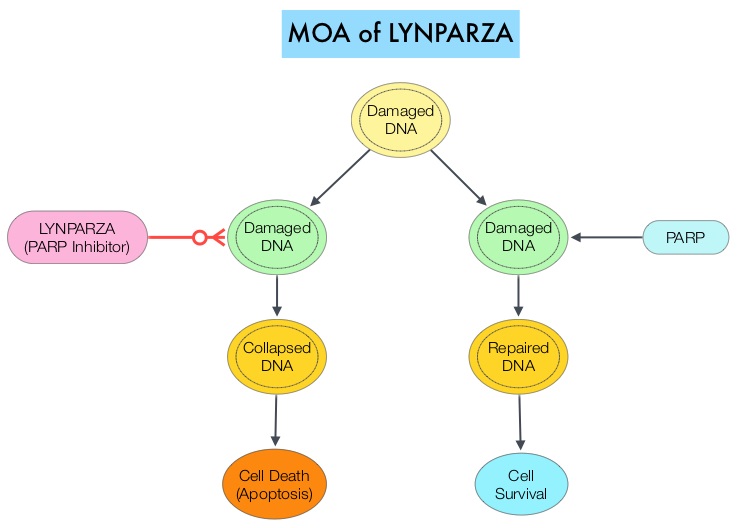
In an attempt to improve outcomes in patients with metastatic PDAC, molecular profiling using Next Generation Sequencing (NGS) and protein IHC panel-based examination of patients’ tumors, has enabled grouping patients into molecular subgroups with therapeutically actionable molecular alterations. It is estimated that approximately 25% of pancreatic cancers harbor actionable molecular alterations, defined as molecular alterations for which there is clinical or strong preclinical evidence of a predictive benefit from a specific therapy. However, less than 5% of patients with pancreatic cancer having actionable molecular alterations receive targeted therapies. This may be because of the aggressive nature of the disease or economic and logistical barriers. The commonly altered pathways include DNA repair (15%), cell cycle (11%), and AKT/mTOR (19%). Molecular targets have included Homologous Recombination Repair genes (14-17%), HER2 amplification genes (2%) and MisMatch Repair gene deficiency (MicroSatellite Instability 2-3%). Mutations in DNA repair genes are the most common “highly actionable” alterations (15%). The most frequently mutated DNA repair genes are ATM (4.5%) and BRCA2 (2.9%). Some examples of available targeted agents for patients with metastatic PDAC include PARP inhibitors for BRCA1 and BRCA2 mutations, TRK inhibitors for NTRK1, NTRK2, or NTRK3 fusions, and Immune Checkpoint Inhibitors for MMR-deficient or MSI-H tumors. Patients with these genetic alterations constitute about 8% of patients, with pancreatic cancer.
Know Your Tumor (KYT) is a precision medicine program, which is a collaboration between Perthera Inc. and the Pancreatic Cancer Action Network (PanCAN), and utilizes Perthera’s precision medicine system for multiomic molecular profiling of a nonselected patient population. Multiomics is data analysis at multiple levels such as genome, epigenome, transcriptome, proteome, and metabolome, to comprehensively understand human health and diseases, by interpreting molecular intricacy and variations. The intent of the KYT program is to match patients with appropriate clinical trials and therapies, based on actionable molecular alterations, treatment history, and geographical locations. The purpose of this study was to determine whether patients with pancreatic cancer whose tumors harbored actionable molecular alterations and who received molecularly matched therapy, had a longer median Overall Survival, than similar patients who did not receive molecularly matched therapy.
In this program, of the 1082 patients who received reports on their tumor genomic profile, outcomes were available for 677 patients, of whom 189 patients had actionable molecular alterations. At a median follow up of 383 days, patients with actionable molecular alterations who received a matched therapy (N=46) had a significantly longer median Overall Survival, compared to those patients who only received unmatched therapies (N=143), and this was statistically significant (2.58 years versus 1.51 years; HR=0.42: P=0.0004). The 46 patients who received a matched therapy also had significantly longer Overall Survival than the 488 patients who did not have an actionable molecular alteration (2.58 years versus 1.32 years; HR=0.34; P<0.0001). The median Overall Survival was not different between the patients who received unmatched therapy and those without an actionable molecular alteration (HR=0.82; P=0.10).
It was concluded from these Real-World outcomes that a matched therapy for patients with actionable molecular alterations can have a substantial effect on survival, in patients with pancreatic cancer. The authors acknowledged that only 2% of patients who were referred to undergo molecular profiling ultimately received a matched therapy and 143 patients with actionable molecular alterations received only unmatched therapies due to a variety of reasons including logistical issues and economic barriers.
Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Pishvaian MJ, Blais EM, Brody JR, et al. Lancet Oncol. 2020 Apr;21(4):508-518.doi: 10.1016/S1470-2045(20)30074-7. Epub 2020 Mar 2.