SUMMARY: The American Cancer Society estimates that over 21,000 women will be diagnosed with Ovarian cancer in the United States for 2015 and over 14,000 will die of the disease. Ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system. The FDA in 2014 approved AVASTIN® (Bevacizumab) in combination with Paclitaxel, Pegylated Liposomal Doxorubicin, or Topotecan, for the treatment of patients with Platinum-resistant, recurrent epithelial Ovarian, Fallopian tube, or Primary Peritoneal cancer. The approval was based on the AURELIA Open-Label Randomized Phase III Trial which concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates, in patients with Platinum Resistant, Recurrent Ovarian Cancer.
ICON7 is an open-label, randomized, phase III trial, in which the safety and efficacy of combining AVASTIN® with standard chemotherapy was evaluated, in patients with Newly Diagnosed Ovarian Cancer. One thousand five hundred and twenty eight (n=1528) patients were enrolled and eligible women with newly diagnosed Ovarian cancer had either early stage disease (FIGO Stage I–IIa, grade 3 or clear cell histology) or more advanced disease (FIGO Stage IIb–IV) disease. Patients had undergone debulking, cytoreductive surgery, or in those with advanced disease, had a biopsy for tissue diagnosis, with no further surgery planned. High risk disease in this study was defined as Stage IV disease, inoperable Stage III disease, or suboptimally debulked (more than 1 cm) Stage III disease. Patients were randomly assigned in a 1:1 ratio to receive either 6 cycles of combination chemotherapy with PARAPLATIN® (Carboplatin) AUC of 5 or 6 and TAXOL® (Paclitaxel) 175mg/m2 IV, given every 3 weeks or the same chemotherapy regimen given concurrently with AVASTIN® (Bevacizumab) 7.5mg/kg IV, every 3 weeks for 6 cycles followed maintenance AVASTIN® given IV every 3 weeks for 12 additional cycles or until disease progression, which ever was the earlier. The median age was 57 years. The Primary endpoint was Progression Free Survival (PFS). Secondary endpoints included Overall Survival and Safety outcomes of adverse events. The median follow up was 48•9 months.
The Primary endpoint of Progression Free Survival (PFS) has been previously reported and was 21•8 months with the addition of AVASTIN® to chemotherapy compared with 20•3 months with chemotherapy alone, in the entire study population (HR=0•81; P=0•004). However, in the predefined high risk population of patients with suboptimally cytoreduced stage III or stage IV disease, the PFS with the addition of AVASTIN® to chemotherapy was 18•1 months versus 14•5 months (HR=0•73; P=0•002).
In this publication, the authors reported the final Overall Survival results of the ICON7 trial. They noted no difference in the Overall Survival between AVASTIN® plus chemotherapy versus chemotherapy alone groups. (45.5 months vs 44.6 months, P=0.85). However, in the predefined group of high risk patients with inoperable or suboptimally cytoreduced stage III or stage IV disease, there was an Overall Survival benefit, with a mean Overall survival of 39•3 months in the AVASTIN® plus chemotherapy group versus 34•5 months in the chemotherapy alone group (P=0•03). This survival benefit was not seen in clear cell, early stage high grade, or low grade serous tumors. It is hypothesized that the effect of AVASTIN® on the tumor microenvironment is dependent on residual tumor burden, which is presumably producing VEGF (Vascular Endothelial Growth Factor). The authors concluded that the Overall Survival benefit with a combination of AVASTIN® and chemotherapy is best accomplished in newly diagnosed Ovarian cancer patients, with poor prognostic factors. Oza AM, Cook AD, Pfisterer J, et al. Lancet Oncol 2015;16:928-936. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial

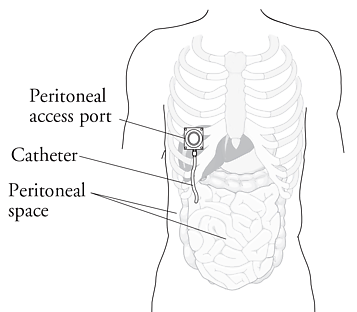 IP chemotherapy for ovarian cancer facilitates the exposure of tumors in the peritoneal cavity to 10-20 fold greater concentration of Cisplatin and Carboplatin and 1000 fold greater concentration of Paclitaxel, compared to IV administration, thus allowing continuous and prolonged exposure of the tumor to high drug concentrations, without systemic toxicities. Even though three Intergroup Phase III trials demonstrated the superiority of IP therapy over IV therapy, it has not been widely accepted in the US and abroad. Barriers to IP therapy have included inconvenience, IP catheter related complications, higher toxicities, lack of knowledge regarding patient selection for IP therapy as well as minimum number of cycles of IP therapy to administer and uncertain long term benefit.
IP chemotherapy for ovarian cancer facilitates the exposure of tumors in the peritoneal cavity to 10-20 fold greater concentration of Cisplatin and Carboplatin and 1000 fold greater concentration of Paclitaxel, compared to IV administration, thus allowing continuous and prolonged exposure of the tumor to high drug concentrations, without systemic toxicities. Even though three Intergroup Phase III trials demonstrated the superiority of IP therapy over IV therapy, it has not been widely accepted in the US and abroad. Barriers to IP therapy have included inconvenience, IP catheter related complications, higher toxicities, lack of knowledge regarding patient selection for IP therapy as well as minimum number of cycles of IP therapy to administer and uncertain long term benefit.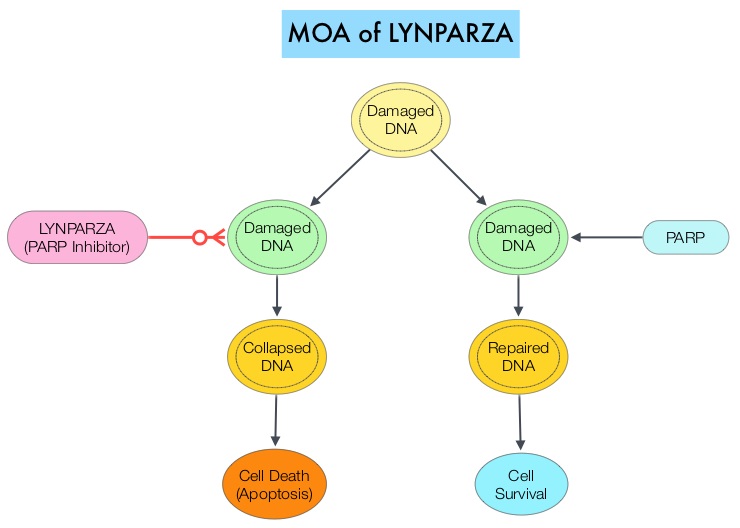 The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The approval of LYNPARZA® was based on a single arm phase II trial in which 137 platinum resistant ovarian cancer patients with measurable germline BRCA mutations were enrolled. The BRCA mutation status was verified retrospectively in 97% of the patients with available blood samples from the phase II study, using the BRACAnalysis CDx® test. These patients had received three or more lines of prior chemotherapy. Treatment consisted of LYNPARZA® administered orally twice a day and was continued until disease progression or unacceptable toxicity. The primary endpoint was Objective Response Rate (ORR). The Overall Response Rate was 34% and the median response duration was 7.9 months. In a larger cohort of patients reported by the authors (ovarian cancer cohort, N=193) the median Progression Free Survival was 7 months, 55% of patients were progression free at 6 months, the median Overall Survival was 16.6 months and 64.4% of patients were alive at 12 months. The most common adverse reactions associated with LYNPARZA® were anemia, nausea, fatigue (including asthenia), vomiting, diarrhea, dysgeusia, dyspepsia, headache, decreased appetite, nasopharyngitis/pharyngitis/URI, cough, arthralgia/musculoskeletal pain, myalgia, back pain, dermatitis/rash and abdominal pain/discomfort. This ground breaking therapy with LYNPARZA® is first of a new class of drugs, for treating ovarian cancer and along with the BRACAnalysis CDx® companion diagnostic test, is a significant milestone for patients with difficult-to-treat advanced ovarian cancer, with germline BRCA mutations. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. [published online November 3, 2014]. J Clin Oncol. doi:10.1200/JCO.2014.56.2728.
The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The approval of LYNPARZA® was based on a single arm phase II trial in which 137 platinum resistant ovarian cancer patients with measurable germline BRCA mutations were enrolled. The BRCA mutation status was verified retrospectively in 97% of the patients with available blood samples from the phase II study, using the BRACAnalysis CDx® test. These patients had received three or more lines of prior chemotherapy. Treatment consisted of LYNPARZA® administered orally twice a day and was continued until disease progression or unacceptable toxicity. The primary endpoint was Objective Response Rate (ORR). The Overall Response Rate was 34% and the median response duration was 7.9 months. In a larger cohort of patients reported by the authors (ovarian cancer cohort, N=193) the median Progression Free Survival was 7 months, 55% of patients were progression free at 6 months, the median Overall Survival was 16.6 months and 64.4% of patients were alive at 12 months. The most common adverse reactions associated with LYNPARZA® were anemia, nausea, fatigue (including asthenia), vomiting, diarrhea, dysgeusia, dyspepsia, headache, decreased appetite, nasopharyngitis/pharyngitis/URI, cough, arthralgia/musculoskeletal pain, myalgia, back pain, dermatitis/rash and abdominal pain/discomfort. This ground breaking therapy with LYNPARZA® is first of a new class of drugs, for treating ovarian cancer and along with the BRACAnalysis CDx® companion diagnostic test, is a significant milestone for patients with difficult-to-treat advanced ovarian cancer, with germline BRCA mutations. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. [published online November 3, 2014]. J Clin Oncol. doi:10.1200/JCO.2014.56.2728.
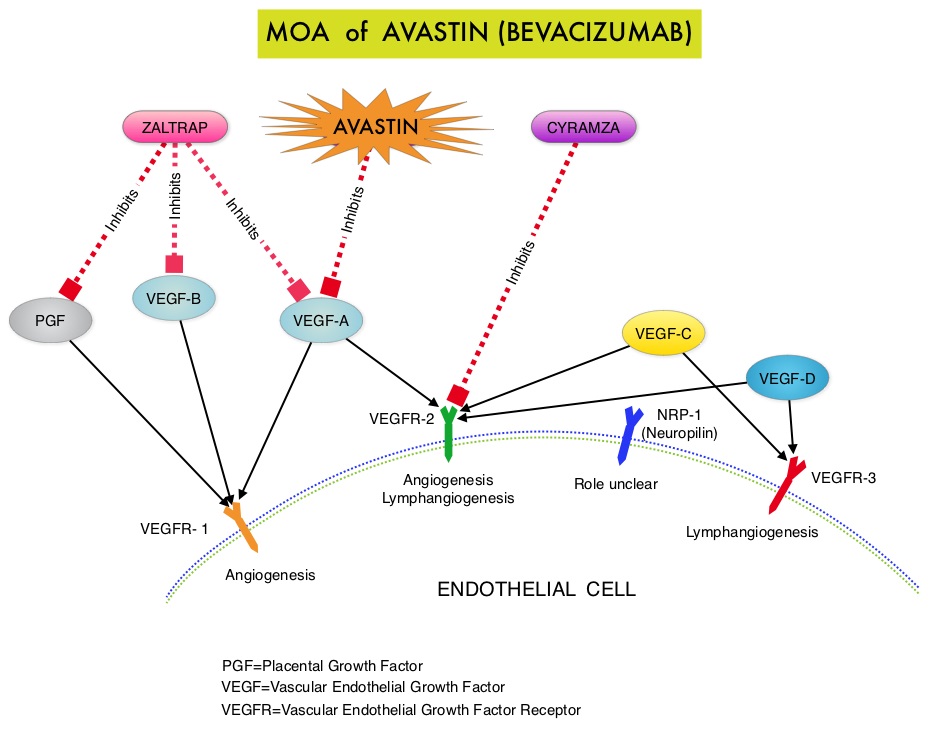 The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 62% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.8 months for the AVASTIN® plus chemotherapy group versus 3.4 months for the single agent chemotherapy group (HR=0.38, P<0.0001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination versus 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination versus 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression received AVASTIN®. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. On exploratory analyses it was noted that the addition of AVASTIN® to TAXOL® resulted in the most benefit, with a 5.7 month improvement in median PFS (9.6 versus 3.9 months), a 23% improvement in the overall response rate (53% versus 30%) and a 9.2 month improvement in median OS (22.4 versus 13.2 months) compared to single agent TAXOL®. This benefit was seen in spite of the fact that 97% of the patients in the TAXOL® group had received this agent with previous chemotherapy regimens. These findings suggest that patients who have received prior treatment with TAXOL® may benefit from AVASTIN® plus weekly TAXOL®. The most common adverse reactions (greater than or equal to 15%) in patients treated with AVASTIN® plus chemotherapy were neutropenia, peripheral neuropathy, hypertension and GI perforation occurred in 1.7% of these patients. This low perforation rate has been attributed to the exclusion of patients with rectosigmoid involvement by pelvic examination or bowel involvement on CT scan as well as those with clinical symptoms of bowel obstruction. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant Recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 62% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.8 months for the AVASTIN® plus chemotherapy group versus 3.4 months for the single agent chemotherapy group (HR=0.38, P<0.0001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination versus 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination versus 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression received AVASTIN®. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. On exploratory analyses it was noted that the addition of AVASTIN® to TAXOL® resulted in the most benefit, with a 5.7 month improvement in median PFS (9.6 versus 3.9 months), a 23% improvement in the overall response rate (53% versus 30%) and a 9.2 month improvement in median OS (22.4 versus 13.2 months) compared to single agent TAXOL®. This benefit was seen in spite of the fact that 97% of the patients in the TAXOL® group had received this agent with previous chemotherapy regimens. These findings suggest that patients who have received prior treatment with TAXOL® may benefit from AVASTIN® plus weekly TAXOL®. The most common adverse reactions (greater than or equal to 15%) in patients treated with AVASTIN® plus chemotherapy were neutropenia, peripheral neuropathy, hypertension and GI perforation occurred in 1.7% of these patients. This low perforation rate has been attributed to the exclusion of patients with rectosigmoid involvement by pelvic examination or bowel involvement on CT scan as well as those with clinical symptoms of bowel obstruction. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant Recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
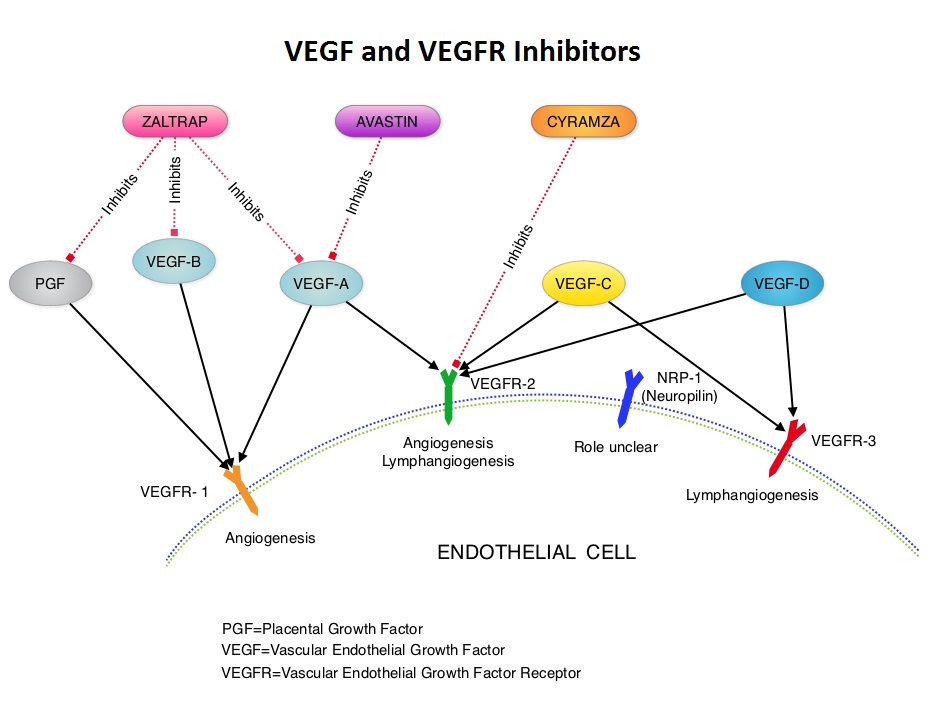 AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308