The FDA on May 27, 2022, granted accelerated approval to KYMRIAH® (Tisagenlecleucel) for adult patients with Relapsed or Refractory Follicular Lymphoma (FL), after two or more lines of systemic therapy. KYMRIAH® is a product of Novartis Pharmaceuticals Corporation.
Tag: Non-Hodgkin Lymphoma
YESCARTA® (Axicabtagene ciloleucel)
The FDA on April 1, 2022, approved YESCARTA® for adult patients with Large B-Cell Lymphoma (LBCL) that is refractory to first-line chemoimmunotherapy or relapses within 12 months of first-line chemoimmunotherapy. It is not indicated for the treatment of patients with primary Central Nervous System lymphoma. YESCARTA® is a product of Kite Pharma, Inc.
Late Breaking Abstract – ASCO 2022: IMBRUVICA® plus Bendamustine and Rituximab for Older Patients with Untreated Mantle Cell Lymphoma
SUMMARY: The American Cancer Society estimates that in 2022, about 80,470 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,250 individuals will die of this disease. In the US, approximately 3,300 new cases of MCL are diagnosed each year. Mantle Cell Lymphoma is an aggressive B-cell lymphoma and accounts for approximately 6% of all Non Hodgkin Lymphomas in adults, and is associated with a high relapse rate following dose-intensive therapies. Early and late relapses in patients with MCL have been attributed to persistence of residual disease.
Majority of patients with MCL are elderly and are not candidates for aggressive treatment or Autologous Stem Cell Transplantation. These patients often receive less aggressive first line therapy such as Bendamustine plus Rituximab, and this regimen has demonstrated superior Progression Free Survival compared to R-CHOP, with a better safety profile. Further, the addition of Rituximab maintenance therapy after induction therapy with Bendamustine and Rituximab has shown significantly prolonged Progression Free Survival or Overall Survival in two independent observational studies.
Bruton’s Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. The 3 BTK inhibitors presently approved by the FDA for MCL include, IMBRUVICA® (Ibrutinib) approved in 2013, CALQUENCE® (Acalabrutinib) approved in 2017, and BRUKINSA® (Zanubrutinib) approved in 2019.
Single agent IMBRUVICA® is presently approved by the FDA for the treatment of MCL patients who have received at least one prior therapy. In a Phase Ib study, the addition of IMBRUVICA® to Bendamustine and Rituximab therapy was safe and effective with a Complete Response Rate of 76%, among patients with untreated, relapsed, or refractory MCL.
SHINE study is an international, randomized, double-blind, Phase III trial , in which a combination of IMBRUVICA® with Bendamustine plus Rituximab and Rituximab maintenance therapy was compared with placebo with Bendamustine plus Rrituximab and Rituximab maintenance therapy, in elderly patients with untreated Mantle Cell Lymphoma (MCL). A total of 523 previously untreated patients, 65 years of age or older, who had a centrally confirmed diagnosis of Mantle Cell Lymphoma with Cyclin D1 overexpression or translocation breakpoints at t(11;14) were randomly assigned in a 1:1 ratio to receive either to six cycles of IMBRUVICA® along with Bendamustine and Rituximab (N=261) or six cycles of placebo along with Bendamustine and Rituximab (N=262). IMBRUVICA® 560 mg or placebo was administered orally once daily. Bendamustine was administered at 90 mg/m2 IV on days 1 and 2 of each 28 day cycle along with Rituximab 375 mg/m2 IV on day 1 of each 28 day cycle. Patients in either arm who achieved a Complete or Partial Response continued to receive IMBRUVICA® or placebo daily along with Rituximab maintenance therapy at a dose of 375 mg/m2 IV every 8 weeks for up to 12 additional doses. Patients with stable disease after induction treatment could continue to receive IMBRUVICA® or placebo until disease progression or unacceptable toxicities. Both treatment groups were well balanced. The median age of the patients was 71 years and eligible patients had documented Stage II to IV disease with at least one measurable site of disease that was at least 1.5 cm in the longest diameter. Patients were excluded if stem-cell transplantation was planned or if they had known CNS involvement. The Primary endpoint was Progression Free Survival (PFS). Secondary endpoints included Objective Response Rate, Complete Response Rate, Overall Survival and Safety.
The study met its Primary endpoint and at a median follow up of 84.7 months, the median PFS was 80.6 months in the IMBRUVICA® group and 52.9 months in the placebo group (HR for disease progression or death=0.75; P=0.01). The Complete Response Rate was 65.5% in the IMBRUVICA® group and 57.6% in the placebo group (P=0.06). The Overall Survival was similar in the two treatment groups. Grade 3 or 4 adverse events during treatment were 81.5% in the IMBRUVICA® group and 77.3% in the placebo group and the most common Grade 3 and 4 adverse events were rash, pneumonia, and atrial fibrillation.
The authors concluded that treatment with IMBRUVICA® in combination with standard chemoimmunotherapy significantly prolonged Progression Free Survival, and may be a new treatment option for elderly patients with newly diagnosed Mantle Cell Lymphoma, who may not be candidates for intensive chemotherapy or Autologous Stem Cell Transplantation.
Ibrutinib plus Bendamustine and Rituximab in Untreated Mantle-Cell Lymphoma. Wang ML, Jurczak W, Jerkeman M, et al. June 3, 2022. DOI: 10.1056/NEJMoa2201817.
DOI: 10.1200/JCO.2022.40.17_suppl.LBA7502 Journal of Clinical Oncology 40, no. 17_suppl (June 10, 2022)
FDA Approves KYMRIAH® for Relapsed or Refractory Follicular Lymphoma
SUMMARY: The FDA on May 27, 2022, granted accelerated approval to KYMRIAH® (Tisagenlecleucel) for adult patients with Relapsed or Refractory Follicular Lymphoma after two or more lines of systemic therapy. The American Cancer Society estimates that in 2022, about 80,470 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,250 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT), Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 22% of all NHLs are Follicular Lymphomas (FL).
Advanced stage indolent NHL is not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life, have been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach. Patients with advanced stage symptomatic Follicular Lymphoma are often treated with induction chemoimmunotherapy followed by maintenance RITUXAN® (Rituximab). This can result in a median Progression Free Survival of 6-8 years. However, approximately 30% of the patients will relapse in 3 years and treatment options are limited for patients with relapses, after multiple treatments. Patients with Follicular Lymphomas often experience a relapsing and remitting pattern of disease and may be exposed to multiple lines of therapy over the course of their disease. In spite of the availability of multiple systemic therapies for Follicular Lymphoma, the efficacy of these regimens drops off rapidly with later lines of therapy. Novel therapies are therefore being investigated to improve outcomes.
Chimeric Antigen Receptor (CAR) T-cell therapy is a type of immunotherapy and consists of T cells collected from the patient’s blood in a leukapheresis procedure, and genetically engineered to produce special receptors on their surface called Chimeric Antigen Receptors (CAR). These reprogrammed cytotoxic T cells with the Chimeric Antigen Receptors on their surface are now able to recognize a specific antigen on tumor cells. These genetically engineered and reprogrammed CAR T-cells are grown in the lab and are then infused into the patient. These cells in turn proliferate in the patient’s body and the engineered receptor on the cell surface help recognize and kill cancer cells that expresses that specific antigen. KYMRIAH® (genetically engineered T-cells) seeks out cancer cells expressing the antigen CD19, which is found uniquely on B cells and destroy them. Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy. Cytokine Release Syndrome, an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab (ACTEMRA®), an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome. The CAR T-cells have been shown to also access sanctuary sites such as the CNS and eradicate cancer cells. CD19 antigen is expressed by majority of the B-cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies.
The present FDA approval was based on the ELARA trial, which is an international, multicenter, single-arm, open-label trial in which the efficacy and safety of KYMRIAH® was investigated in adult patients with Relapsed/Refractory Follicular Lymphoma, after at least two prior therapies. A total of 97 patients received KYMRIAH® (0.6-6×108 CAR+ viable T cells) after lymphodepleting chemotherapy. Bridging therapy was permitted followed by disease assessment prior to KYMRIAH® infusion. Eligible patients had Grades 1-3A Relapsed/Refractory Follicular Lymphoma who had progressed on 2 or more lines of systemic therapy, (including an anti-CD20 antibody and an alkylating agent) or relapsed after Autologous hematopoietic Stem Cell Transplant. The median patient age was 57 years, 85% had Stage III-IV disease, 60% had a FLIPI score 3 or more, 65% had bulky disease, and 42% had LDH above the upper limit of normal. The median number of prior therapies was 4, 78% of patients were refractory to their last treatment and 60% progressed within 2 years of initial anti-CD20 based therapy. The Primary endpoint was Complete Response Rate (CRR) by central review per Lugano 2014 criteria. Secondary endpoints included Overall Response Rate (ORR), Duration of Response (DOR), Progression Free Survival (PFS), Overall Survival (OS), Safety, and cellular kinetics.
In the primary efficacy analysis, with a median follow up 10.6 months, the Overall Response Rate was 86% with a Complete Response Rate of 66%. The response rates were comparable among key high risk subgroups. The median Duration of Response was Not Reached, with 75% of responders still in response at 9 months. At a median follow up of 17 months, the response rates were maintained and the 12-month PFS was 67% and 9 month Duration of Response was 76%. For patients who had a Complete Response, the 12-month PFS was 86% and the estimated Duration of Response was 87%. Approximately 48% of patients experienced Cytokine Release Syndrome (CRS) within eight weeks of infusion, with no patients experiencing CRS of Grade 3 or higher.
It was concluded that after a median follow up of 17 months, KYMRIAH® demonstrated high Response Rates, as well as durable responses, with remarkable safety profile, thus providing a new treatment option for this difficult-to-treat patient group of patients with Relapsed or Refractory Follicular Lymphoma.
Efficacy of Tisagenlecleucel in Adult Patients (Pts) with High-Risk Relapsed/Refractory Follicular Lymphoma (r/r FL): Subgroup Analysis of the Phase II Elara Study. Thieblemont C, Dickinson M, Martinez-Lopez J, et al. Presented in an oral session at the 63rd American Society of Hematology Annual Meeting & Exposition (ASH) 2021:(Abstract #131).
Investigator and Health Systems Insights on Real-World Evidence Associated With a First-Generation BTK Inhibitor in Patients With CLL/SLL
Written by: AJMC® Editorial Staff
Content Sponsored by: BeiGene
Adults experience chronic lymphocytic leukemia (CLL) at a greater rate than they do any other type of leukemia.1 In 2014, ibrutinib became the first Bruton tyrosine kinase (BTK) inhibitor approved by the FDA for the treatment of CLL.2,3 In a 3-year safety study of patients with CLL/small lymphocytic lymphoma (SLL) taking a daily dose of this first-generation medication, ibrutinib was shown to have both a high response rate that increased in quality and frequency over time and modest toxicity.4
However, treatment with ibrutinib has been shown to be associated with adverse events (AEs) that can lead to discontinuation and/or down-dosing.5,6 In particular, atrial fibrillation (AF) is a known AE associated with BTK inhibitor treatment that has been reported in clinical trials.7,8
To determine the economic burden of down-dosing and therapy discontinuation due to AEs after initiation of ibrutinib therapy in patients with CLL/SLL, a team from Milliman, Inc, analyzed 2015 to 2019 data from a proprietary Medicare Advantage claims database that contains annual enrollment information and all Parts A, B, and D claims for approximately 2.5 million annual members.9 Investigators identified patients who developed AF within the first 12 months of starting treatment with ibrutinib. The results of this claims-based analysis were presented during a Science & Innovation Theater presented during the Academy of Managed Care Pharmacy Nexus 2021, held from October 18 to 21, 2021, in Denver, Colorado.9
In the group identified for analysis, investigators examined rates of and average time to discontinuation, down-dosing, and AEs as well as total health care costs accumulated during the 12 months following ibrutinib start.9 Using these key metrics, investigators then compared patients who newly experienced AF (new AF) during this 12-month episode period with those who did not experience new AF during this period. The results of the analysis showed that patients with claims for new AF discontinued ibrutinib at more than twice the rate of patients without claims for new AF and had significantly higher health care utilization.9 These results are explored in detail in a review article, “Real-World Evidence Associated with a First-Generation BTK Inhibitor in Patients With CLL/SLL,” published by The American Journal of Managed Care® (AJMC®) on ajmc.com. In an interview following the review, principal investigator and health care consultant from Milliman, Inc, Kathryn Fitch, RN, MEd, discusses the study’s findings regarding treatment patterns among patients who developed new AF after starting ibrutinib, the costs associated with the development of new AF, and her team’s recent research in this field. In a final interview, Michael Kolodziej, MD, FACP, medical oncologist and Senior Advisor at ADVI Health, LLC, discussed the analysis and its potential implications for managed care.
REFERENCES
1. The American Cancer Society Medical and Editorial Content Team. What is chronic lymphocytic leukemia? American Cancer Society. Updated May 10, 2018. January 13, 2022. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/about/what-is-cll.html
2. Chronic lymphocytic leukemia/small lymphocytic lymphoma: FDA updates. Lymphoma Research Foundation. Updated April 21, 2020. Accessed January 13, 2022. https://lymphoma.org/aboutlymphoma/cll/cllfdaupdates/
3. Center for Drug Evaluation and Research. Approval package for application number 205552Orig2s000. Trade name: Imbruvica. United States Food and Drug Administration. February 12, 2014. Accessed January 13, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205552Orig2s000Approv.pdf
4. Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497-2506. doi:10.1182/blood-2014-10-606038
5. Imbruvica. Prescribing information. Janssen Biotech; 2020. Accessed January 13, 2022. https://www.imbruvica.com/files/prescribing-information.pdf
6. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874-879. doi:10.3324/haematol.2017.182907
7. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805. doi:10.3324/haematol.2017.171041
8. Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14(2):e0211228. doi:10.1371/journal.pone.0211228
9. Fitch KV. Assessing the treatment emergent burden in BTKi therapy: a Medicare analysis in CLL (chronic lymphocytic leukemia). Presented at the Academy of Managed Care Pharmacy Nexus 2021; October 20, 2021; Denver, CO. Accessed January 18, 2022. https://2021.amcpnexus.org/program/science-innovation-theaters
RITUXAN® (Rituximab)
The FDA on December 2, 2021, approved RITUXAN® in combination with chemotherapy for pediatric patients (≥6 months to <18 years) with previously untreated, advanced stage, CD20-positive Diffuse Large B-Cell Lymphoma (DLBCL), Burkitt Lymphoma, Burkitt-Like Lymphoma, or mature B-cell Acute leukemia. RITUXAN® is a product of Genentech, Inc.
FDA Approves YESCARTA® for Second Line Treatment of Large B-cell Lymphoma
SUMMARY: The FDA on April 1, 2022, approved YESCARTA® (Axicabtagene ciloleucel) for adult patients with Large B-cell lymphoma (LBCL) that is refractory to first-line chemoimmunotherapy or relapses within 12 months of first-line chemoimmunotherapy.
What is (CAR) T-cell immunotherapy? Chimeric Antigen Receptor (CAR) T-cell therapy is a type of immunotherapy and consists of T cells collected from the patient’s blood in a leukapheresis procedure, and genetically engineered to produce special receptors on their surface called Chimeric Antigen Receptors (CAR). These reprogrammed cytotoxic T cells with the Chimeric Antigen Receptors on their surface are now able to recognize a specific antigen on tumor cells. These genetically engineered and reprogrammed CAR T-cells are grown in the lab and are then infused into the patient. These cells in turn proliferate in the patient’s body and the engineered receptor on the cell surface help recognize and kill cancer cells that expresses that specific antigen. It is a therefore a customized treatment created using patient’s own T cells to destroy cancer cells.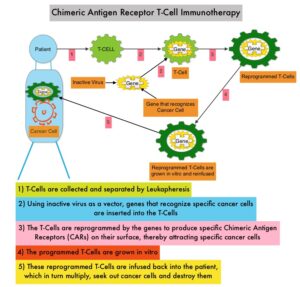
YESCARTA® is a Chimeric Antigen Receptor (CAR) T cell immunotherapy and consists of autologous T cells that are genetically modified to produce a CAR protein, allowing the T cells to seek out and destroy cancer cells expressing the antigen CD19, which is found uniquely on B cells. Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy. Cytokine Release Syndrome (CRS), an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab (ACTEMRA®), an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum Ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome. The CAR T-cells have been shown to also access sanctuary sites such as the central nervous system and eradicate cancer cells. CD19 antigen is expressed by majority of the B cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies such as Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL) and Non Hodgkin lymphoma (NHL), such as Diffuse Large B-Cell Lymphoma (DLBCL).
Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3 to 4% each year. Outcomes for patients with relapsed/refractory disease, is poor, with an Objective response Rate (ORR) of 26%, Complete Response (CR) rate of 8% and a median Overall Survival (OS) of 6.6 months. There is therefore a significant unmet need in this patient group.
The present FDA approval was based on ZUMA-7, an international, randomized, open-label, multicenter Phase III trial, which compared the safety and efficacy of YESCARTA® with that of the current Standard of Care, as second-line treatment in patients with relapsed or refractory Large B-Cell Lymphoma (LBCL). In this study, 359 enrolled patients were randomized 1:1 to receive either a single infusion of YESCARTA® following Fludarabine and Cyclophosphamide lymphodepleting chemotherapy (N=180) or Standard of Care investigator-chosen second-line therapy, consisting of 2 or 3 cycles of chemoimmunotherapy followed by high-dose therapy and autologous Hematopoietic Stem Cell Transplantation (HSCT), in patients who attained Complete Remission or Partial Remission (N=179). Patients randomized to YESCARTA® underwent leukapheresis, and then, lymphodepleting chemotherapy with Fludarabine 30 mg/m2/day and Cyclophosphamide 500 mg/m2/day for 3 days, followed by a single infusion of YESCARTA® at 2 × 106 CAR T cells/kg. Corticosteroid bridging therapy was allowed for patients with high disease burden at screening. Lack of response to chemotherapy was the most common reason for not receiving autologous HSCT, and 35% received on-protocol HSCT. Both treatment groups were well balanced. The median patient age was 59 years, 30% of patients were aged 65 years or older, 79% of patients had Stage III/IV disease, 74% of patients were primary refractory to their frontline therapy, 16% had high-grade B-cell lymphoma (double/triple-hit), and 44% of patients had elevated LDH levels. Key stratification factors included response to frontline therapy and second-line Age-Adjusted International Prognostic Index (sAAIPI) stage. The Primary endpoint was Event Free Survival (EFS), defined as time from randomization to disease progression, start of new lymphoma therapy, or death, and was determined by an Independent Review Committee (IRC). Secondary endpoints included Objective Response Rates (ORR), Overall Survival (OS), Progression Free Survival (PFS), Duration of Response, Safety, and Patient Reported Outcomes (PRO).
At a median follow-up of 24.9 months, the EFS was significantly longer in the YESCARTA® group and the estimated median EFS was 8.3 months in the YESCARTA® group compared with 2.0 months for those receiving Standard of Care chemotherapy (HR=0.40; P<0.0001). The estimated 18-month EFS rate was 41.5% in the YESCARTA® group and 17.0% in the standard therapy group. The IRC-assessed best ORR was statistically significantly higher in the YESCARTA® arm compared to the standard therapy arm (83% versus 50% respectively) and Complete Response rate was 65% versus 32% respectively. EFS was superior with YESCARTA® over Standard of Care across all key patient subgroups, including age, response to first-line therapy at randomization, second-line Age-Adjusted International Prognostic Index (sAAIPI) stage, and prognostic markers.
The authors concluded that ZUMA-7 met its primary EFS end point, demonstrating statistically significant and clinically meaningful improvement in efficacy with YESCARTA® compared to second-line Standard of Care in relapsed/ refractory Large B-Cell Lymphoma, with a 4-fold greater median EFS, a 33% higher Objective Response Rate, a doubling of the Complete Response rate, Event Free Survival improvements across key subgroups, and should therefore be a new standard for patients with second-line relapsed/refractory Large B-Cell Lymphoma. The NCCN updated its clinical practice guidelines to include YESCARTA® as a Category 1 recommendation for patients with early relapsed or primary-refractory Diffuse Large B-Cell Lymphoma.
Primary analysis of ZUMA 7: a phase 3 randomized trial of axicabtagene ciloleucel (axi-cel) versus standard of care therapy in patients with relapsed/refractory large B-cell lymphoma. Locke F, Miklos DB, Jacobson CA, et al. Blood. 2021;138(suppl 1):2. doi:10.1182/blood-2021-148039
BRUKINSA® (Zanubrutinib)
The FDA on September 14, 2021, granted accelerated approval to BRUKINSA® for adult patients with relapsed or refractory Marginal Zone Lymphoma (MZL) who have received at least one anti-CD20-based regimen. BRUKINSA® is a product of BeiGene.
POLIVY® in Previously Untreated Diffuse Large B-Cell Lymphoma
SUMMARY: The American Cancer Society estimates that in 2021, about 81,560 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,720 individuals will die of this disease. Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3-4% each year. More than half of patients are 65 or older at the time of diagnosis and the incidence is likely to increase with aging of the American population. The etiology of Diffuse Large B-Cell Lymphoma is unknown. Contributing risk factors include immunosuppression (AIDS, transplantation setting, autoimmune diseases), UltraViolet radiation, pesticides, hair dyes, and diet.
DLBCL is a neoplasm of large B cells and the most common chromosome abnormality involves alterations of the BCL-6 gene at the 3q27 locus, which is critical for germinal center formation. Two major molecular subtypes of DLBCL arising from different genetic mechanisms have been identified, using gene expression profiling: Germinal Center B-cell-like (GCB) and Activated B-Cell-like (ABC). Patients in the GCB subgroup have a higher five year survival rate, independent of clinical IPI (International Prognostic Index) risk score, whereas patients in the ABC subgroup have a significantly worse outcome. Regardless, R-CHOP regimen (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone), given every 21 days, for 6 cycles, delivered with curative intent, is the current standard of care for patients of all ages, with newly diagnosed DLBCL, regardless of molecular subtype. Approximately 30-40% of patients experience disease progression or relapse, during the first 2 years and attempts to improve on R-CHOP regimen have not been successful. Maintenance treatment strategy following R-CHOP, to better control the disease, delay disease progression and improve long term survival, have included Autologous Stem Cell Transplantation, maintenance treatment with agents such as oral protein kinase inhibitor Enzastaurin and Everolimus. Outcomes for transplant-ineligible patients with Relapsed/Refractory DLBCL patients remain poor.
CD79b is a B-cell specific surface protein, which is a component of the B-cell receptor and is ubiquitously expressed on the surface of malignant B cells. POLIVY® (Polatuzumab vedotin) is a CD79b-directed Antibody-Drug Conjugate (ADC) with activity against dividing B cells. It consists of three components: 1) the humanized ImmunoGlobulin G1 (IgG1) monoclonal antibody specific for human CD79b; 2) the small molecule anti-mitotic agent MMAE (monomethyl auristatin E) and 3) a protease-cleavable linker that covalently attaches MMAE to the Polatuzumab antibody. Upon binding to CD79b, POLIVY® is internalized, and the linker is cleaved by lysosomal proteases thus enabling intracellular delivery of MMAE. MMAE then binds to microtubules and kills dividing cells by inhibiting cell division and inducing apoptosis. POLIVY® demonstrated efficacy in patients with Relapsed or Refractory DLBCL, resulting in significantly longer Overall Survival when combined with Bendamustine and Rituximab, compared to Bendamustine and Rituximab alone. Based on these finding, the FDA granted accelerated approval to POLIVY® in June 2019.
In a Phase Ib-II study POLIVY® in combination with Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (pola-R-CHP) resulted in a 89% Overall Response rate and 77% Complete Responses when given as first line therapy, in patients with DLBCL. In this study, Vincristine was excluded from the regimen owing to the risk of overlapping neurotoxicities with POLIVY®. The present POLARIX trial was conducted to evaluate the efficacy and safety of pola-R-CHP as compared with R-CHOP, in patients with previously untreated DLBCL.
The POLARIX is a randomized, double-blind, placebo-controlled, International Phase III trial in which a total of 879 treatment naïve, CD20-positive, intermediate or high-risk DLBCL patients were randomly assigned in a 1:1 ratio to receive 6 cycles of either pola-R-CHP (N=440) or R-CHOP (N=439). Patients on Day 1 of each 21 day cycle, received POLIVY® 1.8 mg/kg IV and a placebo matching Vincristine IV (pola-R-CHP group) or a placebo matching POLIVY® and intravenous Vincristine at a dose of 1.4 mg/m2 (maximum of 2 mg) (R-CHOP group), along with Rituximab 375 mg/m2 IV, Cyclophosphamide 750 mg/m2 IV and Doxorubicin 50 mg/m2 IV. All the patients also received Prednisone 100 mg orally once daily on Days 1-5 of each of the first six cycles. During cycles 7 and 8, patients in both treatment groups received Rituximab monotherapy at 375 mg/m2 IV. The median patient age was 65 years and stratification was based on IPI score and presence or absence of bulky disease, Subtypes of DLBCL were centrally evaluated and were balanced between the two treatment groups. Patients were eligible regardless of the Cell of Origin or the presence of rearrangements in MYC, BCL2, BCL6, or a combination of these. Patients with known CNS involvement were excluded but CNS prophylaxis with intrathecal chemotherapy was permitted, in accordance with institutional practice guidelines. The use of Granulocyte Colony-Stimulating Factor (G-CSF) was required during the first six cycles of treatment for primary prophylaxis against neutropenia and consolidative radiotherapy to initial sites of bulky disease or extranodal sites was allowed at the discretion of the investigator. The Primary end point was Progression Free Survival (PFS). Secondary end points included Overall Survival (OS) and Safety.
At a median follow up of 28.2 months, the PFS was significantly higher in the pola-R-CHP group compared to the R-CHOP group. The PFS at 2 years was 76.7% in the pola-R-CHP group versus 70.2% in the R-CHOP group (stratified HR=0.73; P=0.02). Treatment with pola-R-CHP resulted in a risk of disease progression, relapse, or death that was 27% lower, compared to R-CHOP. Patient subgroups that did not show a clear benefit with pola-R-CHP included patients 60 years of age or younger, patients with the Germinal Center B-cell-like subtype of DLBCL, patients who had bulky disease, and patients who had lower IPI scores. Overall Survival at 2 years did not differ significantly between the treatment groups and the researchers attributed the lack of a significant difference between the two groups in Overall Survival, to the availability of new, effective treatments for relapsed or refractory DLBCL, as well as short duration of follow up at the time of this reporting. The safety profile was similar in the two treatment groups.
The authors concluded that among patients with previously untreated intermediate-risk or high-risk DLBCL, the risk of disease progression, relapse, or death was lower among those who received pola-R-CHP than among those who received R-CHOP.
Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. Tilly H, Morschhauser F, Sehn LH, et al. December 14, 2021. DOI: 10.1056/NEJMoa2115304
Non Hodgkin Lymphoma Treated with B-Cell Depleting Immunotherapy May Result in Prolonged Hospital Stay and Higher Morbidity after Covid-19 Infection
SUMMARY: The SARS-CoV-2 Coronavirus (COVID-19) induced pandemic first identified in December 2019 in Wuhan, China, has contributed to significant mortality and morbidity in the US, and the number of infections, continue to exponentially increase worldwide. Majority of the patients present with treatment-resistant pyrexia and respiratory insufficiency, with some of these patients progressing to a more severe systemic disease and multiple organ dysfunction.
Patients with lymphoproliferative disorders may be immune deficient due to their underlying disease or due to the therapies they receive, which in turn can increase the incidence and severity of infections. Patients with Non Hodgkin Lymphoma are often treated with CD20 targeted, B-cell depleting monoclonal antibodies such as RITUXAN® (Rituximab) or GAZYVA® (Obinutuzumab), as they were shown to improve survival among patients with B-cell Non-Hodgkin Lymphoma. Depleting B cells dampens the body’s ability to generate antibody responses to new pathogens, which may impact the clinical course of COVID-19. The authors in this study analyzed the clinical course of COVID-19 infection in hospitalized lymphoma patients, and characterized the determinants of worse outcomes.
It has been shown in several studies and registries that patients with hematologic malignancies including lymphomas have a higher incidence of death from COVID-19 compared with other types of cancer. Additional risk factors for COVID-19-related mortality include older age and relapsed or refractory disease. To better understand the risk factors associated with worse outcomes from COVID-19 in this patient population, the authors conducted a retrospective study of 111 patients with lymphoma, hospitalized for COVID-19, at any of the 16 French hospitals, during March and April 2020. The researchers specifically focused on identifying factors associated with prolonged hospital stay (longer than 30 days), or hospitalization for recurrent symptoms for more than 30 days and death, and used length of hospital stay as a proxy for persistent COVID-19 infection. Study patients included those formerly treated for lymphoma, those currently undergoing treatment, or had no treatment.
Of the 111 patients included in this study, 57% (N=63) had previously received B-cell-depleting therapy. The most common type of lymphoma was Diffuse Large B-Cell Lymphoma. Twenty nine percent (29%) of all patients required a prolonged hospital stay (longer than 30 days) due to severe COVID-19 symptoms and persistent disease. The median age of patients with persistent COVID-19 was 64 years and 63% were male. More than two-thirds (69%) had at least one significant comorbidity. None of the patients with T-cell lymphoma included in the study (N=8) experienced persistent COVID-19 infection.
At a median follow-up of 191 days, the 6-month Overall Survival for the entire cohort was 69%. Older age (70 years and over) as well as relapsed/refractory disease were both associated with worse survival and prolonged hospital stays. After adjusting for age, comorbidities, and the presence of relapsed/refractory disease, the researchers noted that receipt of B-cell-depleting treatment within the previous 12 months nearly doubled the likelihood of a prolonged hospital stay and more than doubled the risk of death. After 1 month, 41% of patients who received anti-CD20 monoclonal antibodies were still hospitalized for COVID-19 versus 13% not treated with those antibodies.
The authors concluded that standardized guidelines on the use of anti-CD20 therapies are needed to help us make decisions during the COVID-19 pandemic, and convalescent plasma may be a treatment consideration for B-cell-depleted patients with persistent COVID-19. Patients who recently received B-cell depleting therapies and have COVID-19 should be closely monitored. Additionally, the efficacy and timing of vaccination in this particular population needs further study.
Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Dulery R, Lamure S, Delord M, et al. Am J Hematol. 2021;96:934-944.
