The FDA on August 16, 2018 granted accelerated approval to OPDIVO® for patients with metastatic Small Cell Lung Cancer (SCLC) with progression after platinum-based chemotherapy and at least one other line of therapy. OPDIVO® is a product of Bristol-Myers Squibb Company Inc.
Tag: Lung Cancer: Small Cell
FDA Approves OPDIVO® for Metastatic Small Cell Lung Cancer
SUMMARY: The FDA on August 16, 2018, granted accelerated approval to OPDIVO® (Nivolumab), for patients with metastatic Small Cell Lung Cancer (SCLC) with progression after platinum-based chemotherapy and at least one other line of therapy. Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Small cell lung cancer (SCLC) accounts for approximately 13-15% of all lung cancers and is aggressive. Patients with SCLC are often treated with platinum based chemotherapy as first-line treatment and the tumor response rates are as high as 60-80%. However, only 20% of patients with Limited Stage SCLC are cured and majority of the patients relapse within months of completing initial therapy. The only FDA-approved agent for recurrent or progressive SCLC (second-line treatment) is HYCAMTIN® (Topotecan) and there is presently no standard therapy, after failure on second-line therapy. The 5 year survival rate for Extensive Stage SCLC is less than 5%, with a median survival of 9 to 10 months from the time of diagnosis.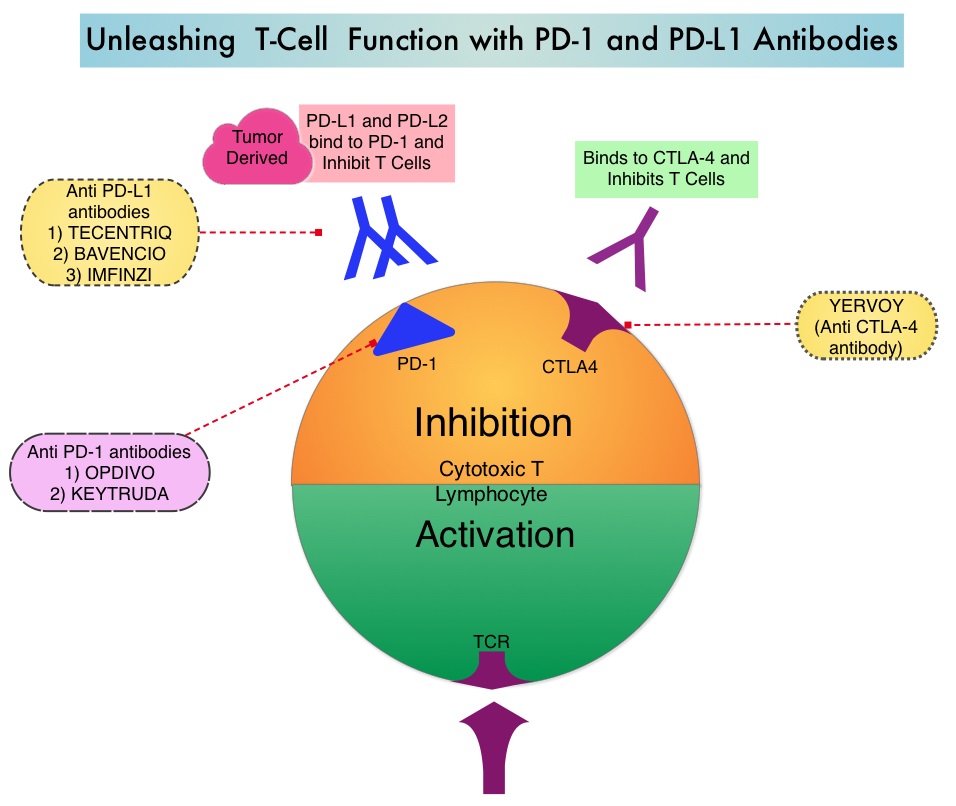
OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor (Checkpoint proteins) and blocks its interaction with PD-L1 and PD-L2. Blocking the Immune checkpoint proteins unleashes the T cells, resulting in T cell proliferation, activation and a therapeutic response.
The present FDA approval for OPDIVO® was based on the results of phase I/II CheckMate-032 trial, which is a multicenter, open-label, ongoing study. This study included 245 patients with SCLC who experienced disease progression after platinum-based chemotherapy. Efficacy data was submitted by the investigators to the FDA from 109 patients who received OPDIVO® after disease progression on platinum-based chemotherapy and at least one other prior line of therapy, to support this present indication. Patients received OPDIVO® 3 mg/kg IV every 2 weeks until disease progression or unacceptable toxicity. The first tumor assessments were conducted 6 weeks after the first dose and were continued every 6 weeks for the first 24 weeks and every 12 weeks thereafter. The Primary endpoint of the study was Objective Response Rate (ORR). Secondary outcome measures included Overall Survival (OS), Progression Free Survival (PFS), Duration of Response (DOR), and the occurrence of treatment-related Adverse Events (AEs) leading to treatment discontinuation.
The results from a blinded, independent central review showed that the ORR was 12% and among the responders, the median Duration of Response was 17.9 months. The responses were durable for 6 months or longer in 77%, 12 months or longer in 62%, and 18 months or longer in 39% of the responding patients. These treatment responses were noted regardless of PD-L1 expression. OPDIVO® was granted accelerated approval for this indication on the basis of Overall Response Rate and Duration of Response, and further proof of benefit in confirmatory trials may be required for full approval.
It was concluded that OPDIVO® is the first new agent approved in nearly 20 years for Small Cell Lung Cancer, and is the first checkpoint inhibitor approved for this patient group. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617370.htm
Late Breaking Abstract – ASCO 2016 ROVA-T, First Targeted Treatment for Small Cell lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and the American Cancer Society estimates that for 2016, about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Small cell lung cancer (SCLC) accounts for approximately 13-15 percent of all lung cancers and is aggressive. The five year survival rate for extensive stage SCLC is less than 5% with a median survival of 9 to 10 months from the time of diagnosis. Patients are often treated with chemotherapy and radiation in the first and second line setting. The Overall Response Rate (ORR) in the third line setting is approximately 18% and the one year Overall Survival is approximately 12%. These patients typically have a poor prognosis with limited treatment options. Delta-like protein 3 also known as DLL3, is encoded by the DLL3 gene and is expressed on the surface of tumor cells but not in normal adult tissues. Patients with high-grade pulmonary NeuroEndocrine Tumors, Small Cell Lung Cancer (SCLC) and Large Cell NeuroEndocrine Carcinoma (LCNEC) have increased expression of DLL3 protein (increased expression seen in approximately 80% of the tumors).
Rovalpituzumab Tesirine (Rova-T) is a first-in-class DLL3-targeted Antibody-Drug Conjugate (ADC) comprised of a humanized anti-DLL3 monoclonal antibody, conjugated to a DNA-damaging PyrroloBenzoDiazepine (PBD) dimer toxin. Rova-T delivers the cytotoxin directly to the DLL3-expressing cancer cells while minimizing toxicity to healthy cells.
The authors in this open-label, Phase 1a/1b, multicenter study, included seventy four (N=74) patients with SCLC who had progressed after at least one previous systemic therapy. Previous therapies included Platinum/Etoposide (96%) and radiation therapy (82%). The majority of patients (76%) had extensive disease at presentation, with 28% having CNS metastases. Over 85% of patients had DLL3 expression on 1% or more of tumor cells and 67% of the patients had DLL3 expression on 50% or more of tumor cells (DLL3-high expression). Patients received Rova-T at doses ranging from 0.05 to 0.8 mg/kg every 3 or 6 weeks. The median age was 61 years. The primary endpoints of the study were Overall Response Rate (ORR) and Maximum Tolerated Dose and secondary endpoints included Overall Survival (OS) and Progression Free Survival (PFS).
Rova-T demonstrated an Overall Response Rate of 39% and Clinical Benefit Rate (stable disease or better) of 89%, in patients with recurrent or refractory Small Cell Lung Cancer identified with high expression of DLL3. The one year Overall Survival rate was 32% in the patient group identified with high expression of DLL3. The most common adverse events were rash, fatigue, nausea, decreased appetite, pleural effusion, peripheral edema and thrombocytopenia.
The authors concluded that Rovalpituzumab Tesirine (Rova-T) has significant single-agent anti-tumor activity with manageable toxicity, in recurrent or refractory SCLC, and is the first biomarker-directed therapy to be defined, for the treatment of Small Cell Lung Cancer. Safety and efficacy of single-agent rovalpituzumab tesirine (SC16LD6.5), a delta-like protein 3 (DLL3)-targeted antibody-drug conjugate (ADC) in recurrent or refractory small cell lung cancer (SCLC). Rudin CM, Pietanza MC, Bauer TM, et al. J Clin Oncol 34, 2016 (suppl; abstr LBA8505)
