The FDA on May 20, 2021 approved OPDIVO® for patients with completely resected Esophageal or GastroEsophageal Junction (GEJ) cancer with residual pathologic disease, who have received neoadjuvant chemoradiotherapy. OPDIVO® is a product of Bristol-Myers Squibb Company.
Tag: Gastric and GE Junction Cancer
KEYTRUDA® (Pembrolizumab)
The FDA on May 5, 2021 granted accelerated approval to KEYTRUDA® (Pembrolizumab) in combination with Trastuzumab, Fluoropyrimidine and Platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2 positive Gastric or GastroEsophageal Junction (GEJ) adenocarcinoma. KEYTRUDA® is a product of Merck & Co.
KEYTRUDA® plus Platinum and Fluoropyrimidine
The FDA on March 22, 2021 approved KEYTRUDA® in combination with Platinum and Fluoropyrimidine-based chemotherapy for patients with metastatic or locally advanced Esophageal or Gastroesophageal (GEJ) (tumors with epicenter 1 to 5 centimeters above the GastroEsophageal Junction) carcinoma, who are not candidates for surgical resection or definitive chemoradiation. KEYTRUDA® is a product of Merck Sharp & Dohme Corp.
ENHERTU® (fam-trastuzumab deruxtecan-nxki)
The FDA on January 15, 2021 approved ENHERTU® for adult patients with locally advanced or metastatic HER2-positive Gastric or GastroEsophageal (GEJ) adenocarcinoma who have received a prior Trastuzumab-based regimen. ENHERTU® is a product of Daiichi Sankyo Company.
ENHERTU® Improves Overall Survival in Previously Treated HER2-Positive Gastric Cancer
SUMMARY: The American Cancer Society estimates that in the US, about 27,600 new cases of Gastric cancer will be diagnosed in 2020 and about 11,010 people will die of the disease. It is one of the leading causes of cancer-related deaths in the world. Several hereditary syndromes such as Hereditary Diffuse Gastric Cancer (HDGC), Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer) and Familial Adenomatous Polyposis (FAP) have been associated with a predisposition for stomach cancer. Additionally, one of the strongest risk factor for Gastric adenocarcinoma is infection with Helicobacter pylori (H.pylori), which is a gram-negative, spiral-shaped microaerophilic bacterium.
The Human Epidermal growth factor Receptor (HER) or erbB family of receptors, consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of advanced Gastric and GastroEsophageal (GE) junction cancers, overexpress or have amplification of the HER2 oncogene. These patients often receive first line treatment with a combination of chemotherapy plus anti-HER2 antibody, Trastuzumab, as there is Overall Survival (OS) benefit with this combination regimen. Upon progression, Paclitaxel plus CYRAMZA® (Ramucirumab), an anti-VEGFR-2 antibody is recommended as second-line therapy, regardless of HER2 expression, based on OS and Progression Free Survival (PFS) data for this combination regimen. Trifluridine-tipiracil (LONSURF®) and Immune Checkpoint Inhibitors are treatment options for later lines of therapy and are associated with minimal prolongation in OS. Unlike treatment in metastatic breast cancer, re-treatment with Trastuzumab in combination with various different chemotherapy agents has not shown survival benefit in Gastric cancer. Further, Antibody-Drug Conjugate such as KADCYLA® (ado-trastuzumab emtansine), did not prolong median OS or improve Response Rates compared to chemotherapy, in patients with Gastric cancer who had progressed during or after treatment with Trastuzumab.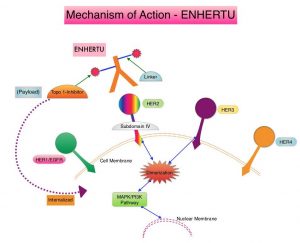
ENHERTU® (Trastuzumab Deruxtecan) is an Antibody-Drug Conjugate (ADC) composed of a humanized monoclonal antibody specifically targeting HER2, with the amino acid sequence similar to Trastuzumab, a cleavable tetrapeptide-based linker, and a potent cytotoxic Topoisomerase I inhibitor as the cytotoxic drug (payload). ENHERTU® has a favorable pharmacokinetic profile and the tetrapeptide-based linker is stable in the plasma and is selectively cleaved by cathepsins that are up-regulated in tumor cells. Unlike KADCYLA®, ENHERTU® has a higher drug-to-antibody ratio (8 versus 4), released payload easily crosses the cell membrane with resulting potent cytotoxic effect on neighboring tumor cells regardless of target expression, and the released cytotoxic agent (payload) has a short half-life, minimizing systemic exposure.
DESTINY-Gastric01 is an open-label, randomized, multicenter, Phase II trial in which ENHERTU® was compared with chemotherapy in patients with HER2-positive advanced Gastric cancer. In this study 187 patients were randomly assigned in a 2:1 ratio to receive either ENHERTU® (N=125) or the treating physician’s choice of Irinotecan or Paclitaxel (N=62). Eligible patients had HER2-expressing advanced Gastric cancer or GastroEsophageal junction adenocarcinoma that had progressed after the receipt of at least two previous systemic therapies, which included a Fluoropyrimidine, a Platinum agent, and Trastuzumab (or approved biosimilar agent). Patients in the ENHERTU® group received the drug at a dose of 6.4 mg/kg as IV infusion every 3 weeks, whereas the physician’s choice group received either Irinotecan monotherapy 150 mg/m2 IV every 2 weeks, or Paclitaxel monotherapy 80 mg/m2 IV on days 1, 8, and 15 every 4 weeks. HER2 levels were documented as high if the score was 3+ on IHC, or 2+ on IHC with positive results on FISH, and documented as low if the score was 2+ on IHC with negative results on FISH, or a score of 1+ on IHC (negative). Treatment was continued until disease progression or unacceptable toxicities. Both treatment groups were well balanced. Approximately 72% of the patients had previously received CYRAMZA® (Ramucirumab), and 86% had received Taxanes. The median time since the last administration of Trastuzumab was 6.2 months. The Primary end point was the Objective Response Rate (ORR), according to Independent Central Review. Secondary end points included Overall Survival (OS), response duration, Progression Free Survival, and safety. The primary cohort consisted of patients with high-level HER2-positive disease, and was the focus of this analysis.
Treatment with ENHERTU® resulted in an ORR of 51%, compared to 14% in the physician’s choice group (P<0.001), according to Independent Central Review. An ORR lasting 4 weeks or more occurred in 43% of patients in the ENHERTU® group, as compared with 12% in the physician’s choice group. More than 80% of patients receiving ENHERTU® had a reduction in tumor size, compared with approximately half the patients receiving physician’s choice of chemotherapy. The median duration of confirmed objective response was 11.3 months in the ENHERTU® group, compared with 3.9 months in the physician’s choice group. Treatment with ENHERTU® resulted in a higher percentage of patients with confirmed disease control (86%), than physician’s choice of chemotherapy (62%). The ORR was higher among those with a HER2 score of 3+ on IHC, than among those with an IHC score of 2+ with positive results on FISH (58% versus 29%).
The Overall Survival was significantly longer in the ENHERTU® group compared to the physician’s choice group (median 12.5 months versus 8.4 months; HR=0.59; P=0.01). The estimated OS at 6 months was 80% in the ENHERTU® group and 66% in the physician’s choice group and at 12 months was 52% and 29%, respectively. In a prespecified subgroup analysis, OS benefit was greater with ENHERTU® compared to physician’s choice of chemotherapy, across most subgroups. The median PFS was 5.6 months in the ENHERTU® group and 3.5 months in the physician’s choice group (HR=0.47). The most common adverse events of Grade 3 or higher were cytopenias. ENHERTU® related Interstitial Lung Disease or pneumonitis was noted in 10% of patients and most events were Grade 1 or 2. Decrease in left ventricular ejection fraction or heart failure was not observed in either treatment groups.
It was concluded that treatment with ENHERTU® resulted in significant improvements in Objective Response Rates and Overall Survival, as compared with standard therapies, among patients with HER2-positive advanced Gastric or GastroEsophageal junction cancer. This benefit was seen even in patients who had disease progression while on Trastuzumab containing regimens.
Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. Shitara K, Bang Y-J, Iwasa S, et al. for the DESTINY-Gastric01 Investigators. N Engl J Med 2020; 382:2419-2430
H. pylori Eradication Treatment Reduces the Risk of Gastric Cancer in High Risk Population
SUMMARY: The American Cancer Society estimates that in the US, about 27,600 new cases of stomach cancer will be diagnosed in 2020 and about 11,010 people will die of the disease. It is one of the leading causes of cancer-related deaths in the world. The incidence of gastric cancer in the US has been on the decline and has been attributed to increased use of refrigeration for food storage, making fresh fruits and vegetables more available, and decreased the use of salted and smoked foods. In the United States, Asians and Hispanics have a much higher incidence of gastric cancer.
Several hereditary syndromes such as Hereditary Diffuse Gastric Cancer (HDGC), Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer) and Familial Adenomatous Polyposis (FAP) have been associated with a predisposition for stomach cancer. HDGC however, is the most common genetic predisposing syndrome for gastric cancer, with germline mutations of the E-cadherin gene (CDH1), detected in 30-50% of diffuse-type gastric cancers. Women with CDH1 mutations are also at an increased risk for breast cancer, and the follow up is similar to BRCA1/BRCA2 mutation carriers. A family history of gastric cancer in a first-degree relative is associated with double to triple the risk of gastric cancer. Gastric cancer overall has a poor prognosis and the 5 year Overall Survival rate is about 25%.
The strongest risk factor for gastric adenocarcinoma is infection with Helicobacter pylori (H.pylori), which is a gram-negative, spiral-shaped microaerophilic bacterium. This bacterial species colonizes the stomach and the overall estimate of H. pylori prevalence in adults is 76% in developing countries and 58% in developed countries. The association between H.pylori and gastric cancer has been shown in observational and case-control studies. In a recently published randomized trial (NEJM 2018;378:1085-1095), treatment of H. pylori infection in patients with early gastric cancer reduced the risk of metachronous gastric cancer by 50%.
It has been unclear whether treatment to eradicate H. pylori can reduce the risk of gastric cancer in individuals with a family history of gastric cancer in first-degree relatives. To address this question, the authors in this single-center, double-blind, placebo-controlled trial, randomly assigned 1838 participants with H. pylori infection and at least one first-degree relative with gastric cancer, in a 1:1 ratio to receive either H.pylori eradication therapy with Amoxicillin 1000 mg, Clarithromycin 500 mg, and Proton-Pump Inhibitor Lansoprazole 30 mg, each taken twice daily for 7 days or placebo. Eligible participants were 40-65 years of age and key exclusion criteria included a history of gastric cancer, peptic ulcer, or other malignancy, and previous eradication therapy for H. pylori. Surveillance endoscopies were performed every 2 years and suspicious lesions were biopsied for gastric cancer. A closeout endoscopy, with H. pylori evaluation, was performed at the end of the trial period. The Primary outcome was development of gastric cancer. A prespecified Secondary outcome was development of gastric cancer according to H. pylori eradication status, assessed during the follow-up period after receipt of H. pylori treatment or placebo. A total of 1676 participants (832 in the treatment group and 844 in the placebo group) were included in the primary outcome analysis.
During a median follow up of 9.2 years, the risk of gastric cancer was 55% lower among those who received H. pylori eradication treatment than among those who received placebo (HR=0.45; P=0.03). Among those who received treatment for H. pylori, the risk of gastric cancer was 73% lower among persons in whom H. pylori eradication was achieved than among those in whom infection was persistent (HR=0.27). Adverse events were common in the treatment group than in the placebo group (53% versus 19.1%; P<0.001), but were mild.
It was concluded that among individuals with H. pylori infection and a family history of gastric cancer in first-degree relatives, H. pylori eradication treatment significantly reduced the risk of gastric cancer. Family History of Gastric Cancer and Helicobacter pylori Treatment. Choi IJ, Kim CG, Lee JY, et al. N Engl J Med 2020;382:427-436
Late Breaking Abstract – ASCO 2019 Front-Line Keytruda® Monotherapy for Patients with Advanced Gastric or Gastroesophageal Junction Adenocarcinoma
SUMMARY: The American Cancer Society estimates that in the US, about 27,510 cases of Gastric Cancer will be diagnosed in 2019 and about 11,140 people will die of the disease. The average age at diagnosis is 68 years and Gastric Cancer is one of the leading causes of cancer-related deaths in the world. Risk factors for gastric cancer include age, gender, ethnicity, geography and infection with Helicobacter pylori. Patients with localized disease (Stage II and Stage III) are often treated with multimodality therapy and 40% of the patients may survive for 5 years or more. However, majority of the patients with Gastric and GastroEsophageal (GE) Adenocarcinoma have advanced disease at the time of initial presentation and have limited therapeutic options with little or no chance for cure. These patients frequently are treated with Platinum containing chemotherapy along with a Fluoropyrimidine and, if appropriate, HER2/neu-targeted therapy. This can however be associated with significant toxicities impacting patient’s quality of life.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response and unleashes the tumor-specific effector T cells. KEYTRUDA® in the Phase II KEYNOTE-059 trial demonstrated promising antitumor activity and durable responses in patients with advanced Gastric/GastroEsophageal Junction cancer, who had progressed on more than 2 lines of therapy, with higher Objective Response Rates noted in patients with PD-L1-positive tumors.
KEYNOTE-062 is a randomized, phase III controlled study in which KEYTRUDA® monotherapy was compared to standard chemotherapy as first line treatment, in select patients with advanced Gastric or GastroEsophageal Junction (GEJ) Adenocarcinoma. This trial enrolled 763 newly diagnosed patients of whom 69% had Gastric Adenocarcinoma cancer and 30% had GEJ Adenocarcinoma. Patients were randomized 1:1:1 to receive KEYTRUDA® 200 mg IV every 3 weeks for up to 2 years (N=256), KEYTRUDA® plus Cisplatin 80 mg/m2 IV every three weeks along with either 5-Fluorouracil 800 mg/m2 daily on Days 1-5 every three weeks or XELODA® (Capecitabine) 1000 mg/m2 twice a day on Days 1-14 every three weeks (N=257 ) or placebo plus Cisplatin and either 5-FU or XELODA® given at a similar dose and schedule as the second group (N=250). The median patient age was 62 years and PD-L1 expression was assessed by Combined Positive Score (CPS). The Primary endpoints were Overall Survival (OS) in patients whose tumors expressed PD-L1 CPS 1 or more and CPS 10 or more in the KEYTRUDA® monotherapy group and in combination with chemotherapy group, as well as Progression Free Survival (PFS) in patients whose tumors expressed PD-L1 CPS 1 or more in the combination arm. Secondary endpoints included Overall Response Rate (ORR) and Duration of Response (DOR) in patients whose tumors express PD-L1 CPS 1 or more. In the current trial, all patients had a PD-L1 CPS of at least 1, and 281 patients (37%) had a PD-L1 CPS score of 10 or more. The median follow-up was 11.3 months.
The trial met its Primary endpoint and among patients with a PD-L1 CPS of 1 or more, Overall Survival was noninferior to chemotherapy. The median Overall Survival was 10.6 months in the KEYTRUDA® monotherapy group compared with 11.1 months in the chemotherapy group (HR=0.91). Among patients with a PD-L1 CPS 10 or more, Overall Survival with KEYTRUDA® was superior to chemotherapy. The median Overall Survival was 17.4 months for those receiving KEYTRUDA® compared with 10.8 months for those receiving chemotherapy. After 2 years, 39% of people taking KEYTRUDA® were alive compared with 22% of those taking chemotherapy (HR=0.69). The OS and PFS rates for the combination of KEYTRUDA® and chemotherapy were comparable with those of chemotherapy alone, regardless of PD-L1 CPS. The efficacy outcomes were not influenced by age, tumor size or location, histological subtype, number of metastatic sites and prior gastrectomy status.
It was concluded that KEYTRUDA® monotherapy is noninferior to chemotherapy for OS among patients with PD-L1 CPS 1 or more. There was however a clinically meaningful improvement in OS among patients with PD-L1 CPS 10 or more. Further, there was a more favorable safety profile for KEYTRUDA® over chemotherapy, making this a more desirable treatment option for elderly patients, for whom platinum based chemotherapy may not be appropriate. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. Tabernero J, Van Cutsem E, Bang Y-J, et al. J Clin Oncol 37, 2019 (suppl; abstr LBA4007)
LONSURF® (Trifluridine/ Tipiracil)
The FDA on February 22, 2019 approved LONSURF® tablets, a fixed combination of Trifluridine, a nucleoside metabolic inhibitor, and Tipiracil, a thymidine phosphorylase inhibitor, for adult patients with metastatic Gastric or GastroEsophageal Junction (GEJ) adenocarcinoma, previously treated with at least two prior lines of chemotherapy that included a Fluoropyrimidine, a Platinum, either a Taxane or Irinotecan, and if appropriate, HER2/neu-targeted therapy. LONSURF® is a product of Taiho Pharmaceutical Co., Ltd.
Late Breaking Abstract – ESMO 2018 LONSURF® Improves Overall Survival in Heavily Pretreated Gastric and GE Junction Cancers
SUMMARY: The American Cancer Society estimates that in the US, about 27,510 cases of Gastric Cancer will be diagnosed in 2019 and about 11,140 people will die of the disease. The average age at diagnosis is 68 years and Gastric Cancer is one of the leading causes of cancer-related deaths in the world. Patients with localized disease (Stage II and Stage III) are often treated with multimodality therapy and 40% of the patients may survive for 5 years or more. However, majority of the patients with Gastric and GastroEsophageal (GE) Adenocarcinoma have advanced disease at the time of initial presentation and have limited therapeutic options with little or no chance for cure. Following progression after first line treatment for metastatic disease, the median survival is approximately 3 months.
LONSURF® (TAS-102) is a combination of two agents – a novel oral nucleoside Trifluridine and a thymidine phosphorylase inhibitor, Tipiracil hydrochloride. This combination has a unique mechanism of action. Trifluridine, the active ingredient of LONSURF® incorporates into DNA resulting in DNA damage. Degradation of Trifluridine which occurs when taken orally is prevented by Tipiracil hydrochloride. In a previously published Phase II study, LONSURF® demonstrated promising efficacy and was well tolerated among pretreated patients with advanced Gastric cancer. TAGS study was conducted to confirm these findings.
The TAGS (TAS-102 Gastric Study) trial is a pivotal Phase III multinational, randomized, double-blind study evaluating LONSURF® (Trifluridine and Tipiracil) plus Best Supportive Care (BSC) versus placebo plus BSC, in patients with metastatic Gastric Cancer, refractory to standard treatments. Enrolled patients (N=507) with histologically confirmed, non-resectable metastatic Gastric cancer including GastroEesophageal junction cancer, who had received 2 or more prior chemotherapy regimens, were randomly assigned in a 2:1 ratio to receive LONSURF® 35 mg/m2 BID on days 1-5 and 8-12 of each 28-day cycle (N=337) or placebo plus Best Supportive Care (N=170). Prior treatments included Fluoropyrimidine, Platinum, Taxane, Irinotecan, or a HER2 inhibitor. Both treatment groups were well balanced and 63% of patients had received 3 or more lines of prior systemic therapy. The Primary endpoint was Overall Survival.
At a median follow-up was 10.7 months, the Primary endpoint was met with a median OS of 5.7 months with LONSURF&rg; versus 3.6 months for those in the placebo group (HR=0.69; P=0.0003). This suggested a 31% reduction in the risk of death when treated with LONSURF®. This benefit was noted across all prespecified subgroups. Treatment with LONSURF® was also associated with 43% lower risk of disease progression or death compared with placebo (HR=0.57; P<0.0001). This Progression Free Survival benefit was again noted in all subgroups. Further, patients in the LONSURF® group had a higher Disease Control Rate (44% versus 14%; P<0.0001) and lower risk of deterioration in Performance Status (HR=0.69; P=0.0005), compared to placebo.
The authors concluded that LONSURF® provided clinically meaningful and statistically significant prolongation in Overall Survival and was well tolerated in patients with heavily pretreated metastatic Gastric Carcinoma. TAGS: a phase 3, randomised, double-blind study of trifluridine/tipiracil (TAS-102) versus placebo in patients with refractory metastatic gastric cancer. Arkenau H-T, Tabernero J, Shitara K, et al. Proceedings from the 2018 ESMO Congress; October 19-23, 2018; Munich, Germany. Abstract LBA25.
