In a multinational double-blind phase III trial, targeting VEGFR-2 signaling with CYRAMZA® , improved overall survival in patients with previously treated advanced or metastatic gastric or gastroesophageal junction adenocarcinoma. CYRAMZA® is the first biological treatment that has demonstrated survival benefit when given as a single agent in this patient population. This study was published in the Lancet, 2014.
Tag: Gastric and GE Junction Cancer
Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD) an international, randomised, multicentre, placebo-controlled, phase 3 trial
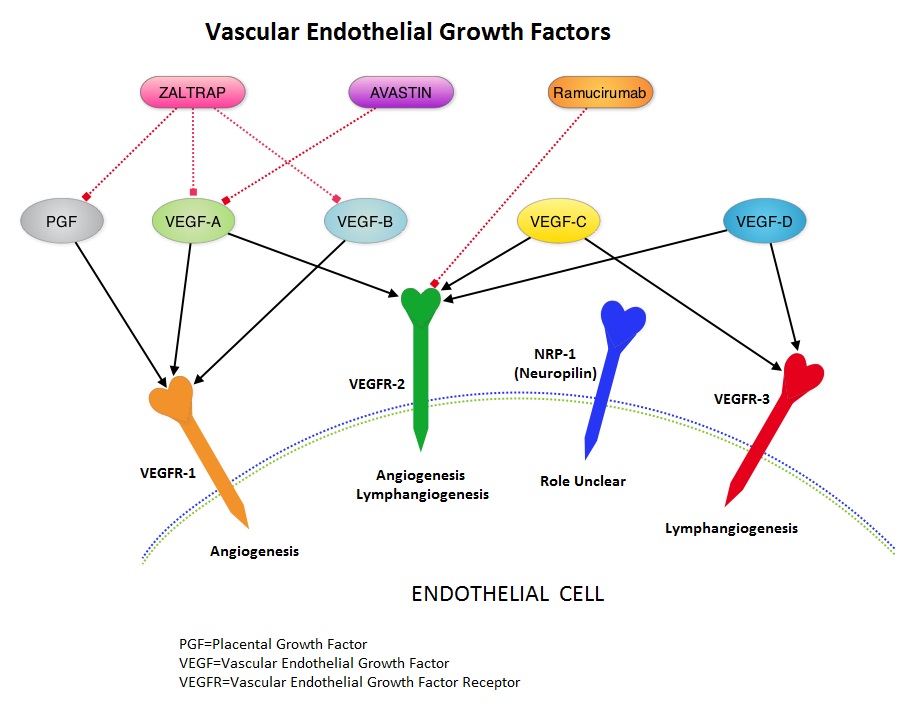
SUMMARY: It is estimated that there were approximately 21,600 new cases and 10,990 deaths from gastric cancer in the United States in 2013. The biology of gastric cancer appears to be different in different parts of the globe. Following progression after first line treatment for metastatic disease, the median survival is approximately 3 months. Ramucirumab (CYRAMZA®) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN® (Bevacizumab) which inhibits VEGF-A. REGARD is a multinational double-blind phase III trial, in which 355 patients with previously treated advanced or metastatic gastric or gastroesophageal junction adenocarcinoma were randomly assigned in a 2:1 ratio to receive CYRAMZA® at 8 mg/kg, IV (N=238) or Placebo (N=117) every 2 weeks. Both groups also received best supportive care. The median age was 60 years and the ECOG performance of the patient population was 0 or 1. Patients received a median of four doses of CYRAMZA® or a median of three doses of Placebo. The primary endpoint was Overall Survival. The median Overall Survival in the CYRAMZA® group was 5.2 months vs 3.8 months in the Placebo group (HR = 0.78, P=0.047). The median Progression-Free Survival was also prolonged in the CYRAMZA® group (2.1 vs 1.3 months, HR = 0.48, P <0 .001). The most common adverse events of any grade in the CYRAMZA® group were hypertension, diarrhea, headache and hyponatremia. The authors concluded that VEGFR-2 signaling is an important therapeutic target in advanced gastric cancer and CYRAMZA® is the first biological treatment that has demonstrated survival benefit when given as a single agent in patients with advanced gastric or gastroesophageal junction adenocarcinoma, progressing after first-line chemotherapy. Fuchs CS, Tomasek J, Yong CJ, et al. Lancet. 2014;383:31-39
Ramucirumab (CYRAMZA®) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN® (Bevacizumab) which inhibits VEGF-A. REGARD is a multinational double-blind phase III trial, in which 355 patients with previously treated advanced or metastatic gastric or gastroesophageal junction adenocarcinoma were randomly assigned in a 2:1 ratio to receive CYRAMZA® at 8 mg/kg, IV (N=238) or Placebo (N=117) every 2 weeks. Both groups also received best supportive care. The median age was 60 years and the ECOG performance of the patient population was 0 or 1. Patients received a median of four doses of CYRAMZA® or a median of three doses of Placebo. The primary endpoint was Overall Survival. The median Overall Survival in the CYRAMZA® group was 5.2 months vs 3.8 months in the Placebo group (HR = 0.78, P=0.047). The median Progression-Free Survival was also prolonged in the CYRAMZA® group (2.1 vs 1.3 months, HR = 0.48, P <0 .001). The most common adverse events of any grade in the CYRAMZA® group were hypertension, diarrhea, headache and hyponatremia. The authors concluded that VEGFR-2 signaling is an important therapeutic target in advanced gastric cancer and CYRAMZA® is the first biological treatment that has demonstrated survival benefit when given as a single agent in patients with advanced gastric or gastroesophageal junction adenocarcinoma, progressing after first-line chemotherapy. Fuchs CS, Tomasek J, Yong CJ, et al. Lancet. 2014;383:31-39
CYRAMZA® (Ramucirumab)
The FDA on April 21, 2014 approved CYRAMZA® for use as a single agent, for the treatment of patients with advanced or metastatic, gastric or gastroesophageal junction (GEJ) adenocarcinoma, with disease progression on or after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy. CYRAMZA® injection for intravenous infusion is a product of Eli Lilly and Company.
RAINBOW A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE)
SUMMARY: It is estimated that there were approximately 21,600 new cases and 10,990 deaths from gastric cancer in the United States in 2013. The biology of gastric cancer appears to be different in different parts of the globe. Following progression after first line treatment for metastatic disease, the median survival is approximately 3 months. Ramucirumab is a human IgG1 monoclonal antibody that inhibits VEGF-receptor 2, unlike AVASTIN® (Bevacizumab) which inhibits VEGF-A. The RAINBOW study is an international, placebo-controlled, double-blind, phase III trial in which 665 patients with metastatic gastroesophageal junction or gastric adenocarcinoma, who had disease progression on or within 4 months after first-line platinum and fluoropyrimidine-based combination therapy, were included. Patients were randomly assigned to receive TAXOL® (Paclitaxel) 80 mg/m2 given on D1, 8, 15 along with Placebo (N=335) or the same dose and schedule of TAXOL® given along with Ramucirumab 8 mg/kg IV every 2 weeks (N=330), of a 28 day cycle. Treatment was continued until disease progression or unacceptable toxicities were noted. The primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR) and Time To Progression (TTP). The median OS for the combination of Ramucirumab and TAXOL® was 9.6 months compared to 7.4 months for Placebo and TAXOL® (P=0.016; HR=0.81), resulting in a 19% reduction in the risk of death with the Ramucirumab and TAXOL® combination. The secondary endpoints favored the Ramucirumab and TAXOL® combination as well. The median PFS was 4.4 months and 2.9 months (P<0.0001; HR=0.63), ORR was 28% and 16% (P<0.0001) and median TTP was 5.5 months and 3 months with the Ramucirumab and TAXOL® combination vs Placebo and TAXOL® combination respectively. As one would expect, treatment related adverse events were seen more frequently in the Ramucirumab and TAXOL® combination group. Significant were neutropenia, hypertension, fatigue and asthenia. The incidence of febrile neutropenia in the two treatment groups was however comparable (3.1% vs 2.4%). The authors concluded that the combination of Ramucirumab and TAXOL® significantly improved both progression-free and overall survival, with significantly improved disease control rates, in patients with metastatic gastroesophageal junction or gastric adenocarcinoma. Wilke H, Van Cutsem E, Oh SC, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA7)