SUMMARY: The FDA on May 1, 2017, granted accelerated approval to IMFINZI® (Durvalumab) for the treatment of patients with locally advanced or metastatic Urothelial Carcinoma who have disease progression during or following platinum-containing chemotherapy or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA also approved the VENTANA PD-L1 (SP263) Assay as a complementary diagnostic for the assessment of the PD-L1 protein in Formalin-Fixed, Paraffin-Embedded Urothelial Carcinoma tissue. Urothelial Carcinoma accounts for 90% of all bladder cancers and can originate in the renal pelvis, ureter and urethra. The American Cancer Society estimates that in 2017, approximately 79,030 new cases of Bladder Cancer will be diagnosed and 16,870 patients will die of the disease. Treatment options for patients who progress after platinum based chemotherapy are limited, with poor outcomes. The response rates with standard chemotherapy in this patient population, is about 10%.
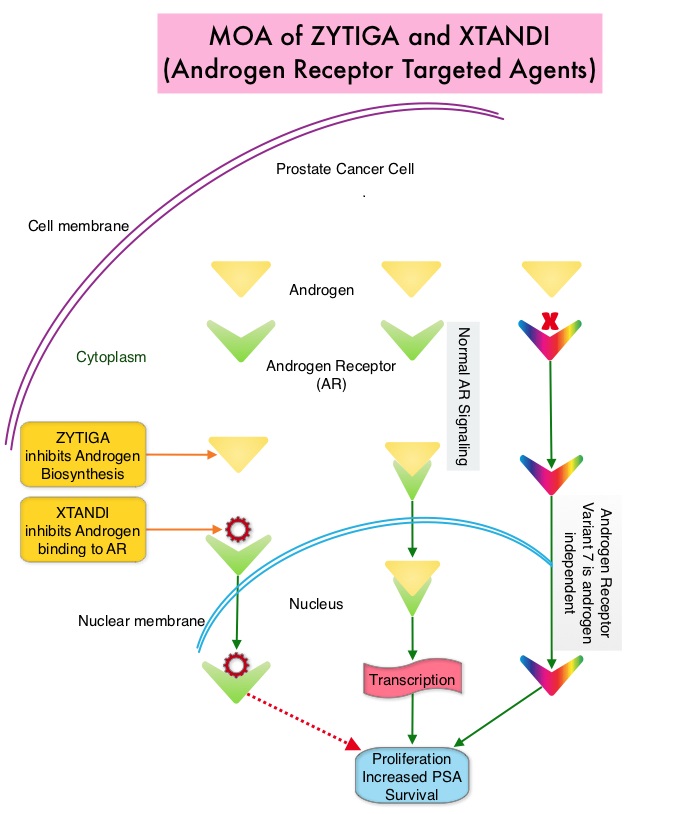
The treatment paradigm for solid tumors has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1) that are expressed by cells in the tumor micro environment. By inhibiting checkpoint proteins and their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response. It has been noted that PD-L1 is widely expressed in tumor and immune cells of patients with Urothelial Carcinoma. This in turn helps cancer cells to evade detection from the immune system by binding to the PD-1 receptor on cytotoxic T lymphocytes.
IMFINZI® is a selective, high-affinity human IgG1 monoclonal antibody directed against PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80. The accelerated FDA approval of IMFINZI® was based on evaluation of 182 patients in the bladder cancer cohort of Study 1108, which is a single-arm Phase I/II trial. This study evaluated the safety and efficacy of IMFINZI® in patients with locally-advanced or metastatic Urothelial Carcinoma of the bladder, who had progressed while on or after a platinum-containing chemotherapy regimen. This study included patients who had progressed within 12 months of receiving therapy, in a neoadjuvant or adjuvant setting. Treatment consisted of IMFINZI® 10 mg/kg IV every 2 weeks until disease progression or unacceptable toxicity. Tumor PD-L1 expression was assessed using the validated VENTANA SP263 Assay, in Formalin-Fixed, Paraffin-Embedded Urothelial Carcinoma tissue. High PD-L1 expression was defined as 25% or more expression on tumor and immune cells. The Primary endpoints were Objective Response Rate and Safety. Secondary endpoints included Duration of Response (DoR) and Overall Survival (OS).
The confirmed Objective Response Rate as assessed by blinded Independent Central Review was 17% and the median response duration was not reached. Objective Response Rate was also analyzed by PD-L1 expression status. In the 182 patients, the confirmed Objective Response Rate was 26.3% in patients with a high PD-L1 score and 4.1% in patients with a low or negative PD-L1 score. The most common adverse reactions were fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, and urinary tract infection.
The authors concluded that IMFINZI® has significant clinical activity and an excellent safety profile in patients with locally advanced or metastatic Urothelial Carcinoma. Clinical trials are underway, evaluating IMFINZI® as monotherapy and in combination with other checkpoint inhibitors, in the first line treatment of patients with advanced bladder cancer. Updated Efficacy and Tolerability of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma. Powles T, O’Donnell PH, Massard C, et al. J Clin Oncol. 2017;35 (suppl 6S; abstract 286).