SUMMARY: In the landmark Prostate Cancer Prevention Trial (PCPT), Finasteride (PROSCAR®) reduced the risk of Prostate Cancer development and therefore the symptoms associated with it by 33%, compared to Placebo. However, in those who did develop Prostate Cancer while on PROSCAR®, there was an increased risk of high grade tumors. After 18 years of follow up of these patients, it appears that in spite of this set back, there was no difference in the overall survival between the PROSCAR® group and the placebo group. The U.S. Preventive Services Task Force (USPSTF) has been against Prostate Cancer screening, as the consensus is that majority of the Prostate Cancers detected by screening would never become apparent in an individual’s lifetime, if this individual was not screened and therefore would never cause a problem. This long term data begs a very important question – Is it worthwhile taking PROSCAR® for Prostate Cancer prevention? Thompson IM, Goodman PJ, Tangen CM, et al. N Engl J Med 2013;369:603-610
Author: RR
Prostate Cancer – Is Prevention Worthwhile?
In the landmark Prostate Cancer Prevention Trial (PCPT), Finasteride (PROSCAR®) reduced the risk of Prostate Cancer development and therefore the symptoms associated with it by 33%, compared to placebo. However, in those who did develop Prostate Cancer while on PROSCAR®, there was an increased risk of more aggressive disease. After 18 years of follow up of these patients (NEJM 2013), it appears that in spite of this set back, there was no difference in the overall survival between the PROSCAR® group and the placebo group. The U.S. Preventive Services Task Force (USPSTF) has been against Prostate Cancer screening, as the consensus is that majority of the Prostate Cancers detected by screening would never become apparent in an individual’s lifetime, if this individual was not screened and therefore would never cause a problem. This long term data begs a very important question – Is it worthwhile taking PROSCAR® for Prostate Cancer prevention?
Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma
SUMMARY: Multiple Myeloma (MM) is a clonal disorder of plasma cells in the bone marrow. It evolves from a precursor stage called Monoclonal Gammopathy of Unknown Significance (MGUS) to MM. Smoldering Multiple Myeloma (SMM) is an intermediate stage in this process of disease evolution. The risk of MGUS transforming into MM is approximately 1% per year. SMM or asymptomatic MM is a precursor to MM and is characterized by at least 10% plasma cells in the bone marrow or a M-spike of at least 3 g/dl, or both, but these patients have no evidence of active symptomatic Myeloma with associated end-organ damage such as hypercalcemia, renal insufficiency, anemia or bone lesions. Even though only 10% of patients with SMM progress to MM annually, over 50% of the SMM patients with high risk features will progress to MM in the first 2 years. The current recommendations for those with SMM are periodic monitoring and treatment intervention only when disease progresses to MM. SMM patients with high risk features include those with at least 10% plasma cells in the bone marrow, a Monoclonal component (IgG monoclonal spike of at least 3g/dL, IgA M-spike of at least 2g/dL or a urinary Bence Jones protein level of more than 1g per 24 hours) or only one of the above two criteria plus at least 95% abnormal plasma cells in the bone marrow, with a reciprocal decrease in one or two uninvolved immunoglobulins of more than 25%, compared to normal values. Identifying those who are at a high risk for progression in the SMM group, is becoming more relevant with the availability of new promising therapies. In a phase III study, 119 patients with high risk SMM were randomly assigned to receive treatment (n=57) or to be observed until progression (n=62).Treatment consisted of Lenalidomide (REVLIMID®) 25 mg given on D1-D21 and Dexamethasone (DECADRON®) 20 mg given on D1-D4 and D12-D15 of a 4 week cycle. Patients received 9 cycles of therapy followed by maintenance therapy with REVLIMID® 10 mg given on D1-D21 every four weeks for 2 years. The median follow-up time was 40 months. The primary end point was time to progression to symptomatic disease. Secondary end points included response rate, overall survival, and safety. The median time to progression was significantly longer in the treatment group compared to the observation group (hazard ratio [HR] = 0.18; P< .001). At 3 years, the survival rate was better in the treatment group than in the observation group (94% vs 80% with a 69% reduction in the risk of death. P =0.03). Treatment related toxicities were grade 2 or lower. The authors concluded that treatment intervention for patients with high risk SMM may be of value, by delaying progression to symptomatic MM and extending overall survival. It remains to be seen if treatment intervention for patients with SMM will become the standard of care. It may also be relevant to include cytogenetics in the SMM definition criteria, as treatment intervention for SMM becomes an acceptable practice. Mateos M-V, Hernandez M-T, Giraldo P, et al. N Engl J Med 2013;369:438-447
Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer
SUMMARY: Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha emitter that selectively targets areas of increased bone turnover. It induces double–stranded DNA breaks and has a very limited range path and quickly loses energy within a short distance of its source. This results in less damage to the adjacent healthy tissue. Further, unlike the dreaded Ra 226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays preventing radiation exposure. In a randomized, double-blind phase III trial, 921 patients with Castrate Resistant Prostate Cancer (CRPC) who had progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons, were randomly assigned in a 2:1 ratio to receive either XOFIGO®, with best supportive care or PLACEBO with best supportive care. Patients with visceral metastases were excluded. The primary endpoint was overall survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. There was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. All adverse events were lower in the XOFIGO® group and myelosuppression was minimal. Unlike the bone seeking beta emitters, Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC with bone metastases. Parker C, Nilsson S, Heinrich D, et al. N Eng J Med 2013;369:213-23
LUX-Lung 3 A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations
SUMMARY: GILOTRIF® (Afatinib) is an oral, irreversible blocker of the ErbB family which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. The approval of GILOTRIF® was based on a multi-center, international, open-label, randomized, phase III trial, in which 345 patients with Stage IIIB (wet)/IV lung adenocarcinoma, with tumors demonstrating Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test, were enrolled in a 2:1 ratio. Patients were randomized to receive GILOTRIF® 40 mg orally once daily (n=230) or ALIMTA® (Pemetrexed)/Cisplatin (n=115). Patients were stratified according to EGFR mutation status (exon 19 deletion vs. exon 21 L858R vs. ‘other’) and race (Asian vs. non-Asian). The primary endpoint was Progression Free Survival (PFS). The median PFS in the GILOTRIF® group was 11.1 months and 6.9 months in the chemotherapy group (HR= 0.58, P<0.001). In patients whose tumors demonstrated EGFR mutations, the median PFS was 13.6 months in the GILOTRIF® arm and 6.9 months in the chemotherapy arm (HR= 0.47, P<0.0001). Objective response rates were 50.4% and 19.1% in the GILOTRIF® and chemotherapy groups respectively. There was no statistically significant difference in overall survival between the two treatment groups. The most frequent adverse reactions in the GILOTRIF® group were skin rash, pruritus, stomatitis, diarrhea and decreased appetite. The authors concluded that GILOTRIF® is better than chemotherapy in the first line treatment of EGFR mutant Non Small Cell Lung Cancer patients. However, it remains to be seen if this agent is superior to TARCEVA® (Erlotinib) and IRESSA® (Gefitinib). Yang JC, Shuler M, Yamamoto N, et al. J Clin Oncol 2012;30(18,Suppl):abstract LBA 7500.
GILOTRIF® for EGFR mutation positive Lung Cancer
GILOTRIF® (Afatinib) is an oral, irreversible blocker of the ErbB family which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. The FDA approved GILOTRIF® based on a multicenter, randomized phase III trial (LUX-Lung 3) in which GILOTRIF® trumped chemotherapy when administered to chemonaive patients with EGFR mutation positive Non Small Lung Cancer. We have yet another targeted oral agent besting chemotherapy. This is another milestone in the Lung Cancer treatment paradigm.
GILOTRIF® (Afatinib)
The FDA on July 12, 2013 approved the use of GILOTRIF® tablets for the first-line treatment of patients with metastatic Non-Small Cell Lung Cancer (NSCLC) whose tumors have Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. The FDA also approved THERASCREEN, a test provided by QIAGEN, for the detection of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations. GILOTRIF® is a product of Boehringer Ingelheim Pharmaceuticals, Inc.
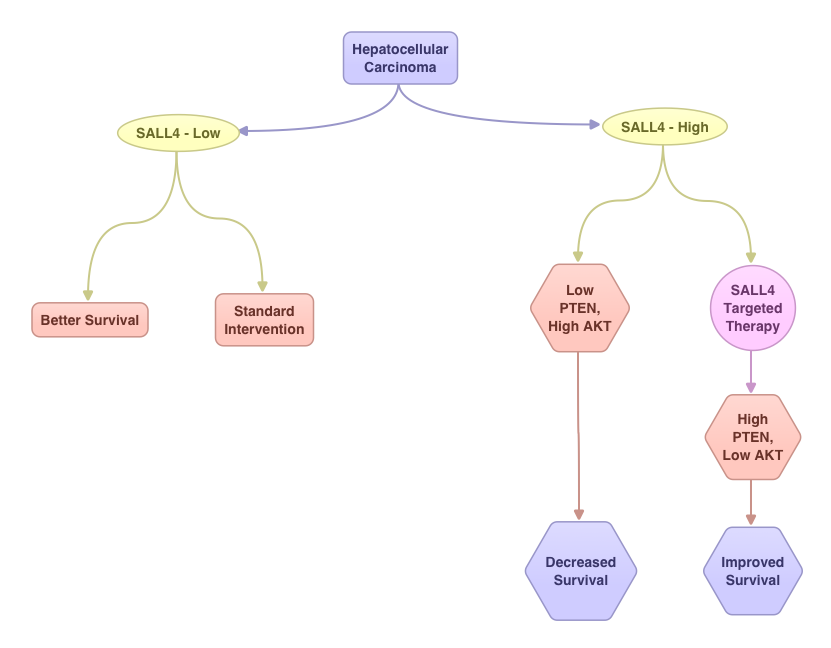
Oncofetal Gene SALL4 in Aggressive Hepatocellular Carcinoma
SUMMARY:Hepatocellular Carcinoma (HCC) originates from hepatocytes and is the sixth most common cancer and third leading cause of cancer related death worldwide. Chronic liver injury and cirrhosis have been implicated as important risk factors. This may result from infections with Hepatitis B and C, heavy alcohol consumption, exposure to Aflatoxin, a potent carcinogen produced by Aspergillus species and non alcoholic fatty liver disease (NAFLD) seen in patients with obesity and diabetes. The underlying liver disease contributing to tumorigenesis adds to the molecular complexity of HCC. The standard intervention for advanced stage HCC has been multi receptor Tyrosine Kinase Inhibitor, NEXAVAR® (Sorafenib). Alpha Feto Protein (AFP) is normally produced by the liver and yolk sac of a fetus during pregnancy and decreases soon after birth. AFP has been used as a serological test for HCC surveillance. In this study, the authors evaluated the role of SALL4, an oncofetal gene, which is expressed in the fetal liver but silenced in the adult liver. In HCC, SALL4 is re-expressed in the tumor tissue and may play an important role in hepatocarcinogenesis and may also portend poor outcomes. SALL4 may therefore serve as an important biomarker and molecular target. Targeting SALL4 could increase the expression of tumor suppressor gene PTEN and block the PI3K survival signaling pathway, by dephosphorylating AKT. The authors concluded that testing hepatic tumor tissue for SALL4 at the time of diagnosis may have prognostic value and may identify patient groups who are likely to benefit from SALL4 targeted therapy. Yong KJ, Gao C, Lim J, et al. N Engl J Med 2013; 368:2266-2276
REVLIMID® (Lenalidomide)
The FDA on June 5, 2013 approved REVLIMID® capsules for the treatment of patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included VELCADE® (Bortezomib). REVLIMID® is a product of Celgene Corporation.
XGEVA® (Denosumab)
XGEVA® (Denosumab): The FDA on June 13, 2013 approved the use of XGEVA® subcutaneous injection, for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity. XGEVA® is a product of Amgen Inc.
