SUMMARY: Prostate cancer is the most common cancer in American men excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 220,800 new cases of prostate cancer will be diagnosed in 2015 and over 27,000 men will die of the disease. The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence.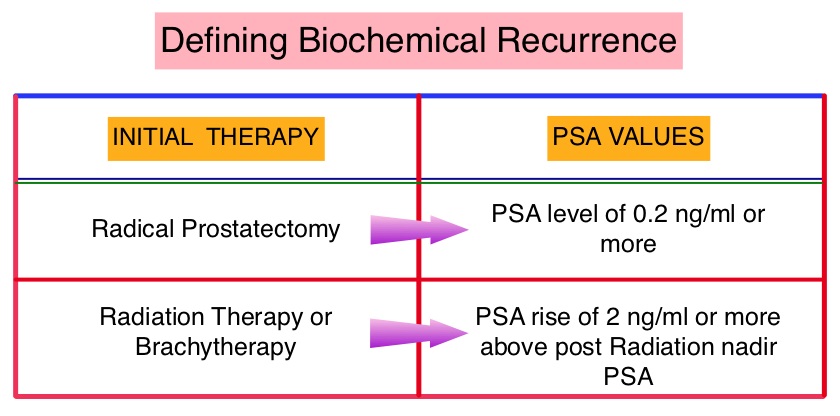 The American Urological Association suggested that a PSA of 0.2 ng/mL or higher after Radical Prostatectomy, defines PSA failure or relapse. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. The appropriate time (immediate versus delayed) to start Androgen Deprivation Therapy (ADT) in patients with prostate cancer with rising Prostate-Specific Antigen (PSA), as the only sign of relapse, has remained unclear. This has been partly due to lack of patient accruals and patient reluctance to be randomized, in these clinical trials.
The American Urological Association suggested that a PSA of 0.2 ng/mL or higher after Radical Prostatectomy, defines PSA failure or relapse. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. The appropriate time (immediate versus delayed) to start Androgen Deprivation Therapy (ADT) in patients with prostate cancer with rising Prostate-Specific Antigen (PSA), as the only sign of relapse, has remained unclear. This has been partly due to lack of patient accruals and patient reluctance to be randomized, in these clinical trials.
The authors conducted this randomized, prospective, phase III trial, to determine if immediate intervention with Androgen Deprivation Therapy (ADT) would improve Overall Survival (OS), compared with delayed ADT, in prostate cancer patients with PSA relapse, following definitive therapy, or in asymptomatic men not suitable for definitive therapy at the time of diagnosis. This analysis combined prostate cancer patients with PSA relapse enrolled in two separate studies. Two hundred and ninety three (N=293) eligible patients were randomly assigned 1:1 to immediate Androgen Deprivation Therapy (N= 142) or delayed ADT (N=151). The primary endpoint was unadjusted Overall Survival. Secondary endpoints included cancer-specific survival and time to clinical progression. The median follow up was 5 years. There was a statistically significant improvement in the Overall Survival, with a 45% reduction in the risk for death, for those receiving immediate ADT compared with the delayed treatment group (HR=0.55; P=0.05). Further, with immediate ADT, there was a statistically significant delay in the time to first local progression (HR= 0.51; P=0.001) as well as time to first metastatic disease (HR=0.54; P=0.018). The authors concluded that immediate Androgen Deprivation Therapy significantly improved Overall Survival and time to clinical progression for prostate cancer patients with PSA relapse, after definitive therapy. This benefit however must be weighed against the risks associated with long term Androgen Deprivation Therapy. TROG 03.06 and VCOG PR 01-03: The “Timing Of Androgen Deprivation therapy in prostate cancer patients with a rising PSA (TOAD)” collaborative randomised phase III trial. Duchesne GM, Bassett J, D’Este C, et al. J Clin Oncol 33, 2015 (suppl; abstr 5007)

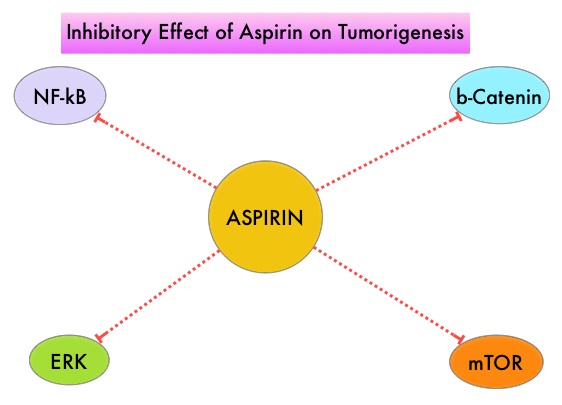 It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties. Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC is unclear. The authors conducted this trial to evaluate the association between Aspirin use after diagnosis of CRC with CRC-Specific Survival (CSS) and Overall Survival (OS) in the largest group of patients ever studied. The study authors in this retrospective study identified 25,644 patients in the Cancer Registry of Norway, diagnosed with ColoRectal Cancer (CRC) from 2004 through 2011. Using the Norwegian Prescription Database, the authors were then able to establish that 6,109 patients in this large cohort had documented exposure to Aspirin. Exposure to Aspirin was defined as a prescription for more than 6 months of Aspirin following a diagnosis of CRC. The median follow up was 2.2 years. The authors performed a multivariate regression analysis controlling for age, gender, tumor stage, tumor differentiation and noted that exposure to Aspirin post-diagnosis, independently improved ColoRectal Cancer (CRC) -Specific Survival (HR=0.75; P<0.001) and Overall Survival (HR=0.86; P<0.001). The authors concluded that in this large group of unselected ColoRectal Cancer (CRC) patients, exposure to Aspirin after the diagnosis of CRC is independently associated with improved Colorectal Cancer-Specific Survival and Overall Survival. They added that because of the risk of bleeding, the risk–benefit should be assessed before Aspirin is routinely recommended to this patient population. Impact of aspirin as secondary prevention in an unselected cohort of 25,644 patients with colorectal cancer: A population-based study. Bains S, Mahic M, Cvancarova M, et al. J Clin Oncol 33, 2015 (suppl; abstr 3504)
It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties. Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC is unclear. The authors conducted this trial to evaluate the association between Aspirin use after diagnosis of CRC with CRC-Specific Survival (CSS) and Overall Survival (OS) in the largest group of patients ever studied. The study authors in this retrospective study identified 25,644 patients in the Cancer Registry of Norway, diagnosed with ColoRectal Cancer (CRC) from 2004 through 2011. Using the Norwegian Prescription Database, the authors were then able to establish that 6,109 patients in this large cohort had documented exposure to Aspirin. Exposure to Aspirin was defined as a prescription for more than 6 months of Aspirin following a diagnosis of CRC. The median follow up was 2.2 years. The authors performed a multivariate regression analysis controlling for age, gender, tumor stage, tumor differentiation and noted that exposure to Aspirin post-diagnosis, independently improved ColoRectal Cancer (CRC) -Specific Survival (HR=0.75; P<0.001) and Overall Survival (HR=0.86; P<0.001). The authors concluded that in this large group of unselected ColoRectal Cancer (CRC) patients, exposure to Aspirin after the diagnosis of CRC is independently associated with improved Colorectal Cancer-Specific Survival and Overall Survival. They added that because of the risk of bleeding, the risk–benefit should be assessed before Aspirin is routinely recommended to this patient population. Impact of aspirin as secondary prevention in an unselected cohort of 25,644 patients with colorectal cancer: A population-based study. Bains S, Mahic M, Cvancarova M, et al. J Clin Oncol 33, 2015 (suppl; abstr 3504) Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab) , an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The U. S. Food and Drug Administration granted approval to OPDIVO®, for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC), with progression on or after platinum based chemotherapy. CheckMate 057 is a randomized, international, phase 3 study designed to evaluate the benefit of OPDIVO® for patients with Non-Squamous (NSQ) NSCLC who had progressed after platinum-based doublet chemotherapy. A total of 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (n=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (n=290). Eligible patients included those with advanced Non-Squamous NSCLC who had progressed after platinum-based doublet chemotherapy and a Tyrosine Kinase Inhibitor (TKI), if deemed eligible for a TKI. Treatment was continued until disease progression or unacceptable toxicity. The primary clinical endpoint was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Progression Free Survival (PFS), Efficacy based on PD-L1 expression, Quality of Life, and Safety. The study was stopped earlier than expected following assessment by the independent Data Monitoring Committee (DMC) which concluded that the study met its endpoint, demonstrating superior overall survival, in patients receiving OPDIVO®, compared to the control group. Patients in the OPDIVO®, group had a significantly higher median OS compared to those in the TAXOTERE® group (12.2 months versus 9.4 months, Hazard Ratio [HR] 0.73, P=0.0015). This meant a 27% reduction in the risk of death in the OPDIVO® group and this survival benefit was seen in all predefined subgroup of patients. The Objective Response Rate (ORR) was also significantly higher for patients receiving OPDIVO® compared to TAXOTERE® (19% versus 12%, P=0.0246) and the median duration of response (DOR) was significantly higher for the OPDIVO® group (17.2 months) vs the TAXOTERE® group (5.6 months). More importantly, when tumor PD-L1 expression was correlated with Overall Survival, the median OS for OPDIVO® was 17.2 months, 18.2 months, and 19.4 months for patients with tumors having 1% or higher, 5% or higher, and 10% or higher of cells staining positive for PD-L1, respectively, compared with 9.0 months, 8.1 months, and 8.0 months with TAXOTERE® treatment. Even though this study showed significant survival outcomes for patients expressing any level of PD-L1, the magnitude of benefit was even more so, in patients with tumors expressing higher levels of PD-L1. PD-L1 expression may therefore be a predictor of response, although this should not yet be used for patient selection. Grade 3-5 adverse events occurred more often in the TAXOTERE® group compared to the OPDIVO® group (54% vs 10%). Based on this compelling data, the authors concluded that OPDIVO® significantly improves Overall Survival when compared to TAXOTERE®, in patients with advanced non-Squamous NSCLC, after failure of platinum based doublet therapy. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Paz-Ares L, Horn L, Borghaei H, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA109)</s
Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab) , an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The U. S. Food and Drug Administration granted approval to OPDIVO®, for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC), with progression on or after platinum based chemotherapy. CheckMate 057 is a randomized, international, phase 3 study designed to evaluate the benefit of OPDIVO® for patients with Non-Squamous (NSQ) NSCLC who had progressed after platinum-based doublet chemotherapy. A total of 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (n=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (n=290). Eligible patients included those with advanced Non-Squamous NSCLC who had progressed after platinum-based doublet chemotherapy and a Tyrosine Kinase Inhibitor (TKI), if deemed eligible for a TKI. Treatment was continued until disease progression or unacceptable toxicity. The primary clinical endpoint was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Progression Free Survival (PFS), Efficacy based on PD-L1 expression, Quality of Life, and Safety. The study was stopped earlier than expected following assessment by the independent Data Monitoring Committee (DMC) which concluded that the study met its endpoint, demonstrating superior overall survival, in patients receiving OPDIVO®, compared to the control group. Patients in the OPDIVO®, group had a significantly higher median OS compared to those in the TAXOTERE® group (12.2 months versus 9.4 months, Hazard Ratio [HR] 0.73, P=0.0015). This meant a 27% reduction in the risk of death in the OPDIVO® group and this survival benefit was seen in all predefined subgroup of patients. The Objective Response Rate (ORR) was also significantly higher for patients receiving OPDIVO® compared to TAXOTERE® (19% versus 12%, P=0.0246) and the median duration of response (DOR) was significantly higher for the OPDIVO® group (17.2 months) vs the TAXOTERE® group (5.6 months). More importantly, when tumor PD-L1 expression was correlated with Overall Survival, the median OS for OPDIVO® was 17.2 months, 18.2 months, and 19.4 months for patients with tumors having 1% or higher, 5% or higher, and 10% or higher of cells staining positive for PD-L1, respectively, compared with 9.0 months, 8.1 months, and 8.0 months with TAXOTERE® treatment. Even though this study showed significant survival outcomes for patients expressing any level of PD-L1, the magnitude of benefit was even more so, in patients with tumors expressing higher levels of PD-L1. PD-L1 expression may therefore be a predictor of response, although this should not yet be used for patient selection. Grade 3-5 adverse events occurred more often in the TAXOTERE® group compared to the OPDIVO® group (54% vs 10%). Based on this compelling data, the authors concluded that OPDIVO® significantly improves Overall Survival when compared to TAXOTERE®, in patients with advanced non-Squamous NSCLC, after failure of platinum based doublet therapy. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Paz-Ares L, Horn L, Borghaei H, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA109)</s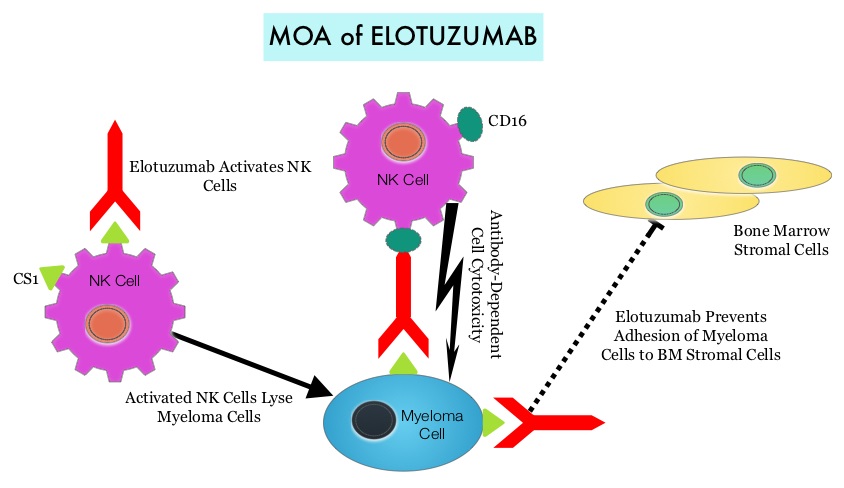 By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s
By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s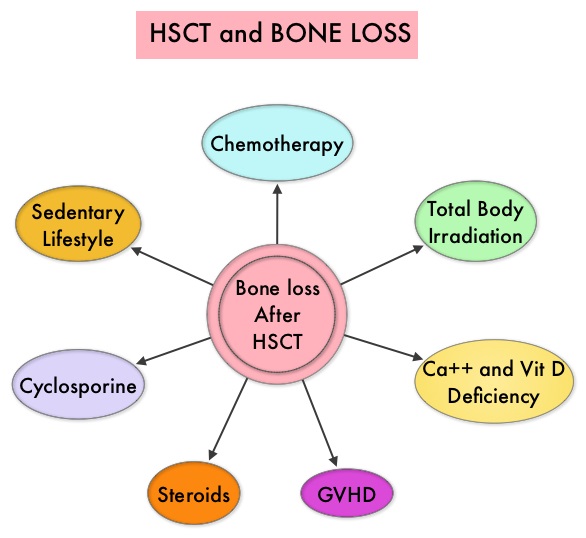 Morbidity and mortality as well as quality of life related to bone loss among long term survivors after HSCT, has been previously published. Factors contributing to bone loss in this patient population include intensive chemotherapy, total body irradiation, post-transplantation glucocorticoid use, reduced intake and metabolism of Calcium and Vitamin D, graft versus host disease, use of Cyclosporine-A and sedentary lifestyle after transplantation. Loss of Bone Mineral Density (BMD) occurs within 6 to 12 months after transplantation at all skeletal sites, followed by initial recovery of BMD in the lumbar spine and a slower recovery of BMD in the femur neck. This bone loss may persist for 48 to 120 months or even longer. The authors in this single institution, retrospective study, calculated the cumulative incidence rates of fractures among survivors of Autologous and Allogeneic Hematopoietic Stem Cell Transplantations (HSCT) and compared the rate of fractures to that of the general US population. Data was collected from 7,620 patients over 18 years of age who underwent HSCT at The University of Texas MD Anderson Cancer Center, from January 1997 to December 2011 and these patients were observed through December 2013. The authors then calculated the cumulative incidence of fractures, with death as a competing risk and the age and sex-specific fracture incidence rates were compared with those in the US general population, using estimated rates from the 1994 National Health Interview Survey and the 2004 National Hospital Discharge Survey. Of the 7,620 patients who underwent HSCT, 56% were male and 51% underwent Autologous and 49% underwent Allogeneic stem cell transplantation.
Morbidity and mortality as well as quality of life related to bone loss among long term survivors after HSCT, has been previously published. Factors contributing to bone loss in this patient population include intensive chemotherapy, total body irradiation, post-transplantation glucocorticoid use, reduced intake and metabolism of Calcium and Vitamin D, graft versus host disease, use of Cyclosporine-A and sedentary lifestyle after transplantation. Loss of Bone Mineral Density (BMD) occurs within 6 to 12 months after transplantation at all skeletal sites, followed by initial recovery of BMD in the lumbar spine and a slower recovery of BMD in the femur neck. This bone loss may persist for 48 to 120 months or even longer. The authors in this single institution, retrospective study, calculated the cumulative incidence rates of fractures among survivors of Autologous and Allogeneic Hematopoietic Stem Cell Transplantations (HSCT) and compared the rate of fractures to that of the general US population. Data was collected from 7,620 patients over 18 years of age who underwent HSCT at The University of Texas MD Anderson Cancer Center, from January 1997 to December 2011 and these patients were observed through December 2013. The authors then calculated the cumulative incidence of fractures, with death as a competing risk and the age and sex-specific fracture incidence rates were compared with those in the US general population, using estimated rates from the 1994 National Health Interview Survey and the 2004 National Hospital Discharge Survey. Of the 7,620 patients who underwent HSCT, 56% were male and 51% underwent Autologous and 49% underwent Allogeneic stem cell transplantation. 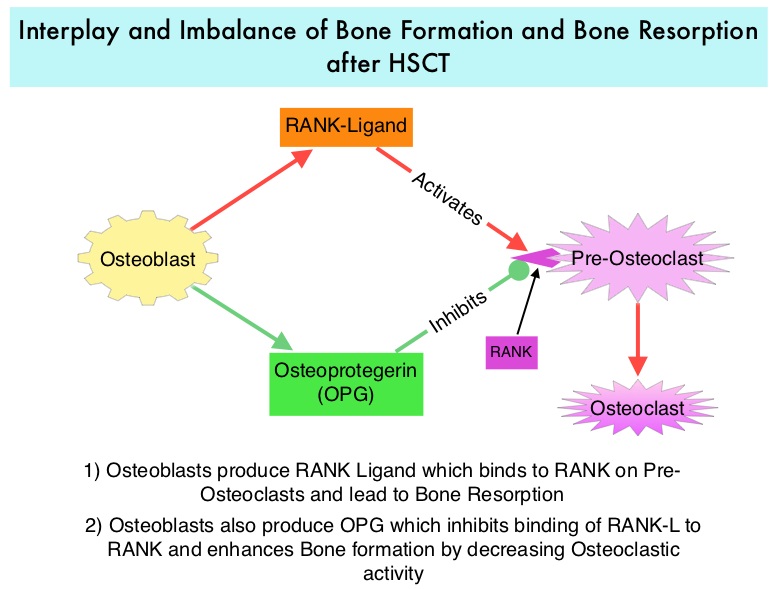 The most common reasons for HSCT were hematologic malignancies other than Multiple Myeloma (67%), Multiple Myeloma (22%) and other solid tumors (11%). The median follow up was 85 months. Fractures occurred in 8% of patients (N = 602) of whom 419 patients had an Autologous stem cell transplantation and 183 patients had Allogeneic stem cell transplantation. The incidence of fracture was higher in patients older than age 50 years, 5 times higher among patients with Multiple Myeloma compared to other hematologic malignancies and patients who underwent Autologous transplantation were 45% more likely to develop a fracture than those who underwent an Allogeneic transplantation. When age- and sex-specific fracture incidence rates after HSCT were compared with National Health Interview Survey data, females were at approximately 8 times greater risk and men 45-64 years old were at approximately 7-9 times greater risk of sustaining a fracture. The authors concluded that the incidence of fractures after HSCT is significantly higher and all patients undergoing HSCT should be considered to be at risk for post-transplantation bone loss. Measures to prevent bone loss and fractures include physical exercise, Vitamin D and Calcium supplementation, avoiding tobacco products, abstaining from excess alcohol intake and fall prevention. The authors recommend that patients undergoing HSCT should have a Dual Energy X-ray Absorptiometry scan performed at baseline and at 6 months following transplantation. Increased Incidence of Fractures in Recipients of Hematopoietic Stem-Cell Transplantation. Pundole XN, Barbo AG, Lin H, et al. J Clin Oncol 2015; 33:1364-1370
The most common reasons for HSCT were hematologic malignancies other than Multiple Myeloma (67%), Multiple Myeloma (22%) and other solid tumors (11%). The median follow up was 85 months. Fractures occurred in 8% of patients (N = 602) of whom 419 patients had an Autologous stem cell transplantation and 183 patients had Allogeneic stem cell transplantation. The incidence of fracture was higher in patients older than age 50 years, 5 times higher among patients with Multiple Myeloma compared to other hematologic malignancies and patients who underwent Autologous transplantation were 45% more likely to develop a fracture than those who underwent an Allogeneic transplantation. When age- and sex-specific fracture incidence rates after HSCT were compared with National Health Interview Survey data, females were at approximately 8 times greater risk and men 45-64 years old were at approximately 7-9 times greater risk of sustaining a fracture. The authors concluded that the incidence of fractures after HSCT is significantly higher and all patients undergoing HSCT should be considered to be at risk for post-transplantation bone loss. Measures to prevent bone loss and fractures include physical exercise, Vitamin D and Calcium supplementation, avoiding tobacco products, abstaining from excess alcohol intake and fall prevention. The authors recommend that patients undergoing HSCT should have a Dual Energy X-ray Absorptiometry scan performed at baseline and at 6 months following transplantation. Increased Incidence of Fractures in Recipients of Hematopoietic Stem-Cell Transplantation. Pundole XN, Barbo AG, Lin H, et al. J Clin Oncol 2015; 33:1364-1370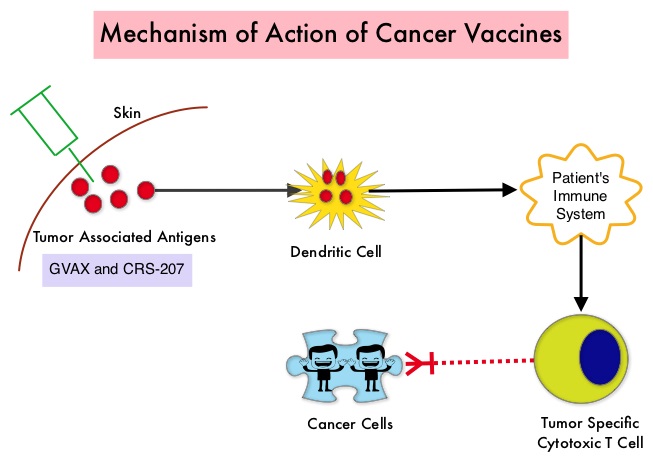 The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333