SUMMARY: The FDA on May 27, 2016 approved AXUMIN® (Fluciclovine F18), a novel molecular radiopharmaceutical diagnostic agent, for Positron Emission Tomography (PET) imaging in men with suspected prostate cancer recurrence, based on elevated Prostate Specific Antigen (PSA) levels, following prior treatment. Prostate cancer is the most common cancer in American men with the exclusion of skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 180,890 new cases of prostate cancer will be diagnosed in 2016 and over 26,000 men will die of the disease.
The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse.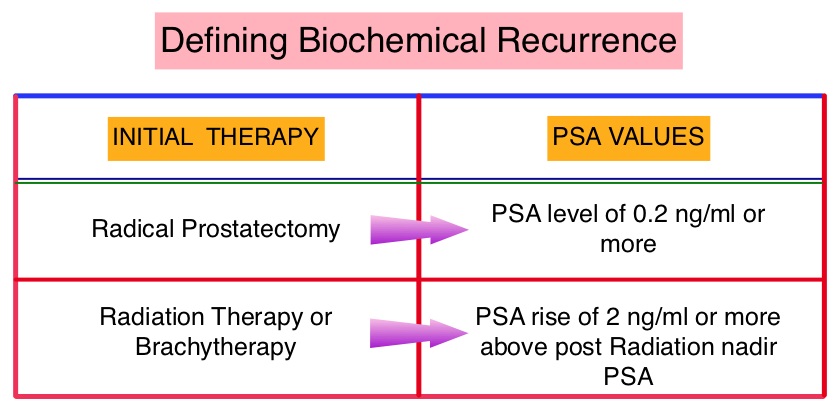
Rising PSA is therefore a sign of recurrent disease and identifying the site of recurrence can be of immense value for the clinician and can help determine the best course of therapy. The diagnostic accuracy of standard imaging tests, for the identification of sites of recurrence in patients with biochemical recurrence, is low. Almost 90% of the standard imaging tests such as CT/MRI and Bone Scan may be negative. More accurate non-invasive imaging techniques for the detection of recurrent tumor is an unmet need. Prostascint, a Single Photon Emission Computerized Tomography (SPECT) radiopharmaceutical agent, was approved in 1999 for the diagnostic imaging of post-prostatectomy patients with a rising PSA. PET scans have largely superseded this study. FluDeoxyGlucose F18 (FDG), a glucose analogue is the most widely used PET radiotracer, but is not generally used as an imaging agent in prostate cancer. This is because good and reliable quality images are not feasible due to indolent growth of prostate cancers and the high urinary excretion of FDG. The other PET radiotracer that is available, Choline C11, has been shown to improve cancer detection in men with biochemical recurrent prostate cancer, but this agent has a short half life of 20 minutes, requires greater patient preparation including 6 hours of fasting prior to administration of Choline C11, delivers higher radiation dose to patients and image quality is poor.
AXUMIN® (Fluciclovine), a diagnostic radiopharmaceutical, is a synthetic amino acid that is preferentially transported into prostate cancer cells by amino acid transporters such as LAT-1 and ASCT2, which are upregulated in prostate cancer cells. This agent is neither metabolized nor incorporated into newly synthesized proteins. The visualization of the increased amino acid transport is facilitated by labeling AXUMIN® with F18 for PET imaging. The FDA approval of AXUMIN® was based on two retrospective trials (Trial 1 and Trial 2) which evaluated the safety and efficacy of AXUMIN® for imaging prostate cancer, in patients with recurrent disease. Trial 1 compared 105 (N=105) AXUMIN® scans in men with suspected prostate cancer, to the histopathology (study of tissue changes caused by disease) obtained by prostate biopsy and by biopsies of suspicious imaged lesions. PET/CT imaging generally included both abdomen and pelvic regions. Local radiologist read the scans initially and subsequently, three independent radiologists read the same scans in a blinded study. Trial 2 evaluated the concordance between 96 (N=96) AXUMIN® and Choline C11 scans, in patients with median PSA values of 1.44 ng/mL. Local radiologist read the Choline C11 scans, and the same three independent radiologists from Trial 1 read the scans, in this second blinded study.
The FDA reported that results of the independent scan readings were generally consistent and confirmed the local scan reading results, and both studies supported the safety and efficacy of AXUMIN® for imaging prostate cancer in men with elevated PSA levels, following prior treatment. It should be noted that a negative study does not rule out the presence of recurrent prostate cancer and a positive image does not confirm the presence of recurrent prostate cancer. Clinical correlation, which may include histopathological evaluation of the suspected recurrence site, is recommended. The most commonly reported adverse events in patients were injection site pain, redness and a metallic taste in the mouth.
It was concluded that AXUMIN® can determine the location of the recurrent prostate cancer in patients with low PSA levels. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208054Orig1s000TOC.cfm