The FDA on May 13, 2016 approved LENVIMA® capsules in combination with AFINITOR® (Everolimus), for the treatment of advanced Renal Cell Carcinoma following one prior anti-angiogenic therapy. LENVIMA® was first approved in 2015 for the treatment of locally recurrent or metastatic, progressive, RadioActive Iodine-refractory Differentiated Thyroid Cancer. LENVIMA® is a product of Eisai, Inc.
Author: RR
CABOMETYX® (Cabozantinib)
The FDA on April 25, 2016 approved CABOMETYX® for the treatment of advanced Renal Cell Carcinoma, in patients who have received prior anti-angiogenic therapy. CABOMETYX® is a product of Exelixis, Inc.
VENCLEXTA® (Venetoclax)
The FDA on April 11, 2016 approved VENCLEXTA® for the treatment of patients with Chronic Lymphocytic Leukemia (CLL) with 17p deletion, as detected by an FDA approved test, who have received at least one prior therapy. VENCLEXTA® tablets are marketed by AbbVie, Inc. and Genentech USA, Inc.
DEFITELIO® (Defibrotide Sodium)
The FDA on March 30, 2016 approved DEFITELIO® for the treatment of adult and pediatric patients with hepatic Veno-Occlusive Disease (VOD), also known as sinusoidal obstructive syndrome, with renal or pulmonary dysfunction, following Hematopoietic Stem Cell Transplantation (HSCT). DEFITELIO® is a product of Jazz Pharmaceuticals, Inc.
XALKORI® (Crizotinib)
The FDA on March 11, 2016 approved XALKORI® capsules for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC) whose tumors are ROS1-positive. XALKORI® is a product of Pfizer, Inc.
AFINITOR® (Everolimus)
The FDA on February 26, 2016 approved AFINITOR® for the treatment of adult patients with progressive, well-differentiated non-functional, neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin with unresectable, locally advanced or metastatic disease. AFINITOR® is a product of Novartis Pharmaceuticals Corporation.
GAZYVA® (Obinutuzumab)
The FDA on February 26, 2016 approved GAZYVA® for use in combination with Bendamustine followed by GAZYVA® monotherapy for the treatment of patients with Follicular Lymphoma (FL) who relapsed after, or are refractory to, a RITUXAN® (Rituximab) containing regimen. GAZYVA® was previously approved for use in combination with Chlorambucil for the treatment of patients with previously untreated Chronic Lymphocytic Leukemia. GAZYVA® injection is a product of Genentech, Inc.
IBRANCE® (Palbociclib)
The FDA on February 19, 2016 approved IBRANCE® in combination with FASLODEX® (Fulvestrant) for the treatment of women with hormone receptor (HR) positive, Human Epidermal growth factor Receptor 2 (HER2)-negative advanced or metastatic breast cancer, with disease progression following endocrine therapy. IBRANCE® capsules are a product of Pfizer, Inc.
HALAVEN® (Eribulin)
The FDA on January 28, 2016 approved HALAVEN® for the treatment of patients with unresectable or metastatic Liposarcoma who have received a prior Anthracycline-containing regimen. HALAVEN® is a product of Eisai Co., Ltd.
ZYPREXA® Combination Significantly Reduces Nausea and Vomiting in Patients Receiving Highly Emetogenic Chemotherapy
SUMMARY: Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. The development of effective antiemetic agents has facilitated the administration of majority of the chemotherapy agents in an outpatient setting avoiding hospitalization. Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment, whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on Serotonin (5-hydroxytryptamine-5HT3) and its receptors, with the chemotherapeutic agents stimulating the release of Serotonin from the enterochromaffin cells of the small intestine. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are also located centrally in the Chemoreceptor Trigger Zone of the area Postrema. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems.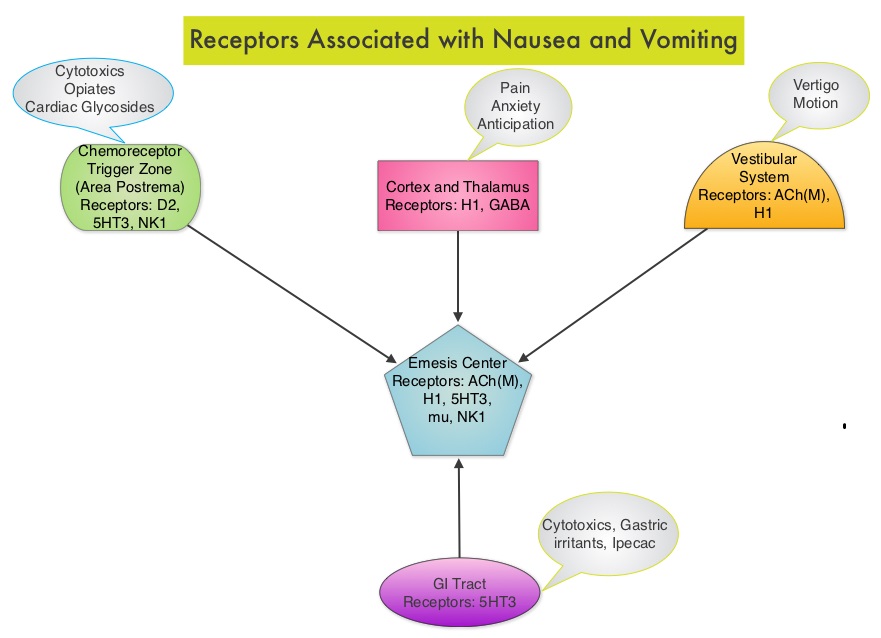
ZYPREXA® (Olanzapine) is an antipsychotic agent that has been shown to block multiple neurotransmitters including Dopamine at D1, D2, D3, and D4 receptors, Serotonin at 5-HT2, 5-HT3 receptors, as well as Catecholamines at alpha1-adrenergic receptors, Acetylcholine at muscarinic receptors and Histamine at H1 receptors in the central nervous system. By virtue of its mechanism of action, ZYPREXA® might have significant antiemetic properties. Based on its pharmacological properties, this study evaluated the efficacy of ZYPREXA® for the prevention of nausea and vomiting, in patients receiving highly emetogenic chemotherapy.
The authors conducted a randomized, double-blind, phase III trial in which patients receiving ZYPREXA® (N=192) were compared to patients receiving Placebo (N=188). Patients received either ZYPREXA® 10 mg PO or matching Placebo daily, on days 1-4 of chemotherapy cycles. All patients additionally received Dexamethasone, Aprepitant or Fosaprepitant, and a 5-HT3 receptor antagonist. Eligible patients had no previous chemotherapy and were receiving Cisplatin at or more than 70 mg/m2 or Cyclophosphamide and Doxorubicin combination. The primary endpoint was nausea prevention and a Complete Response defined as no emesis and no use of rescue medication, was the secondary endpoint.
It was noted that the proportion of patients with no chemotherapy induced nausea was significantly greater with ZYPREXA® than with placebo in the first 24 hours after chemotherapy (74% versus. 45%, P=0.002), the time interval from 25 to 120 hours after chemotherapy (42% versus 25%, P=0.002) and across the overall 120-hour period (37% versus 22%, P=0.002). The Complete Response Rate was also significantly increased with ZYPREXA® during the three periods: 86% versus 65% (P<0.001), 67% versus 52% (P=0.007), and 64% versus 41% (P<0.001), respectively. Sedation, which is a side effect of ZYPREXA®, was observed on day 2 of treatment, with severe sedation noted in 5% of the patients.
The authors concluded that ZYPREXA® significantly improved nausea prevention, as well as Complete Response rate when compared to placebo, among previously untreated patients receiving highly emetogenic chemotherapy. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. Navari RM, Qin R, Ruddy KJ, et al. N Engl J Med 2016; 375:134-142
