SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2017 about 222,500 new cases of lung cancer will be diagnosed and over 155,000 patients will die of the disease. Non Small Cell Lung Cancer accounts for approximately 85% of all lung cancers. The treatment paradigm for malignancies has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers inhibit an intense immune response by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are now available that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1), that are expressed by cells in the tumor micro environment. By targeting the Immune check point proteins or their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.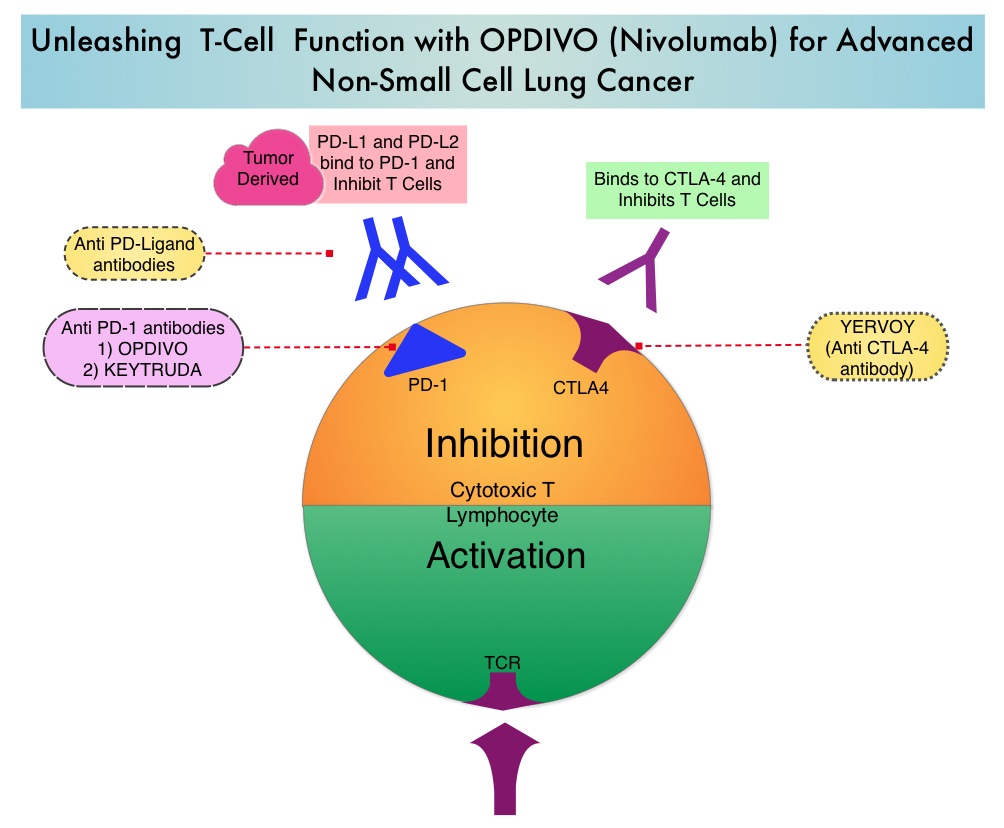
OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. Even though immune checkpoint inhibition has taken center stage in the management of advanced lung cancer as well as a number of other malignancies, the efficacy of these agents appears to vary based on the site of metastatic disease. Previously published studies have shown that in patients with advanced NSCLC, adrenal lesions and lymph nodes were more responsive when treated with OPDIVO®, followed by lung lesions. Liver lesions were less responsive to OPDIVO®. (Nishino M, Ramaiya NH, Chambers ES, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. Journal for Immunotherapy of Cancer. 2016;4:84. doi:10.1186/s40425-016-0193-2).
To address this further, the authors in this publication reviewed the efficacy of OPDIVO® in lung cancer patients with hepatic metastases, at their institution. A retrospective study was conducted and this analysis included data from 75 patients with advanced lung cancer, who were treated with PD1 inhibitor OPDIVO®, at East Carolina University. The study was designed to evaluate predictive markers of response to immune checkpoint blockade. Thirteen percent (13%) of the patients had liver metastases. The median age was 62 years, 22% of the patients had squamous cell, 33% had adenocarcinoma, and 44% had small cell neuroendocrine histology. Patients had an average of 1.7 therapies prior to treatment with OPDIVO®. Patients in this study received an average of 4 cycles of anti-PD1 therapy with OPDIVOreg;. Forty four percent (44%) of the patients received adjunctive therapy such as radiation (33%) or immune modulating chemotherapy, with the aim of augmenting the effect of the anti-PD1 therapy.
It was noted that that none of the patients with hepatic metastases experienced an objective decrease in their liver metastases after treatment with OPDIVO®. These patients had an average survival of 132 days after initial treatment with OPDIVO®.
The authors concluded that this is the largest reported series evaluating lung cancer patients with hepatic metastases, who had been treated with PD-1 inhibitors. They noted that consistent with previously published studies, patients with liver metastases have poor outcomes with anti-PD1 therapy and the mechanisms underlying such resistance must be elucidated, so that more effective treatment combinations can be developed. Outcomes with immune checkpoint inhibitor use in lung cancer patients with hepatic metastases. Addepalli S, Chipman R, Stroud G, et al. J Clin Oncol 35, 2017 (suppl 7S; abstract 38)

