SUMMARY: The American Cancer Society estimates that approximately 137,000 new cases of colorectal cancer will be diagnosed in the United States in 2014 and over 50,000 are expected to die of the disease. It is the third leading cause of cancer death in the U.S. and the lifetime risk of developing colorectal cancer is 1 in 20. Implementation of screening programs in the U.S. has resulted in a 46% decrease in the rate of death from colorectal cancer, from its peak. The U.S. Preventive Services Task Force recommends annual screening with high sensitivity Fecal Occult Blood Testing (FOBT), sigmoidoscopy every 5 years with high-sensitivity FOBT every 3 years and screening colonoscopy every 10 years. Fecal Immunochemical testing (FIT) measures intact human globin protein as opposed to heme. Animal heme from meat will not trigger a false positive test with FIT and as such dietary restrictions are not necessary. Further, FIT is superior to FOBT in detecting advanced adenomas and only requires one stool specimen, as opposed to three specimens for FOBT.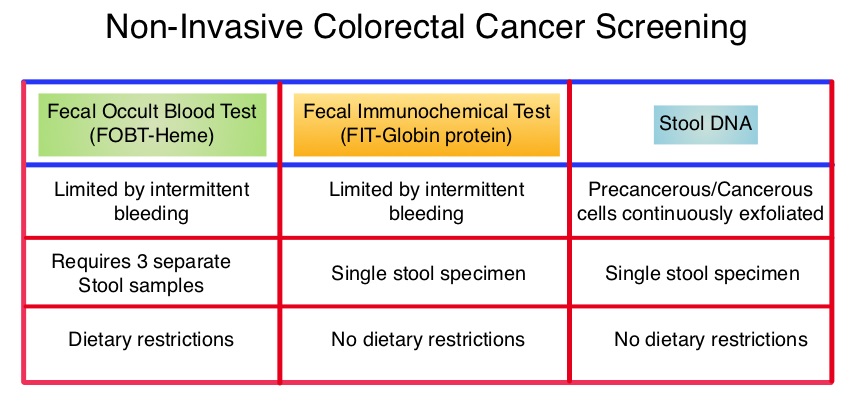 COLOGUARD® is highly sensitive, noninvasive, multitarget, stool based DNA test, for colorectal cancer screening ie.early detection of colorectal cancer and precancerous lesions. This test takes advantage of the genetic and epigenetic alterations that leads to the development of colorectal cancer and analyzes the altered DNA signatures of cancerous and precancerous cells that exfoliate into the colon. In addition, this test includes an immunochemical assay for human hemoglobin. The stool samples can be easily collected, mailed from home, and requires no bowel preparation, medication restrictions or dietary change. The purpose of this study was to determine the sensitivity of COLOGUARD® as compared with FIT, for the detection of screening-relevant colorectal cancer. The study was conducted at 90 medical centers throughout the United States and Canada and evaluable patients (N=9989) were asymptomatic individuals between ages 50 and 84 years who were required to provide a stool sample and undergo screening colonoscopy within 90 days. Enrollment was weighted toward those aged 65 years or older. The primary outcome was the ability of COLOGUARD® to detect colorectal cancer and secondary outcomes were the ability of COLOGUARD® to detect advanced precancerous lesions, polyps with high grade dysplasia and serrated sessile polyps measuring 1 cm or more, when compared to FIT. (Sensitivity is defined as the proportion of persons with disease who have a positive test and Specificity is defined as proportion of persons without disease who have a negative test.) The sensitivity for detecting colorectal cancer was 92% with COLOGUARD® and 74% with FIT (P=0.002). The sensitivity for detecting advanced precancerous lesions was 42.4% vs 23.8% (P<0.001), polyps with high-grade dysplasia was 69.2% vs 46.2% (P=0.004) and serrated sessile polyps measuring 1 cm or more were 42.4% vs 5.1%, (P<0.001) with COLOGUARD® and FIT respectively. Specificities with COLOGUARD® were lower compared to FIT (P<0.001). The authors concluded that COLOGUARD® can detect significantly more colorectal cancers and precancerous lesions than FIT. The high sensitivity of this non-invasive, stool DNA test, to detect curable stage of colorectal cancer, may increase the number of people, who will choose to be screened for colorectal cancer. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. N Engl J Med 2014; 370:1287-1297
COLOGUARD® is highly sensitive, noninvasive, multitarget, stool based DNA test, for colorectal cancer screening ie.early detection of colorectal cancer and precancerous lesions. This test takes advantage of the genetic and epigenetic alterations that leads to the development of colorectal cancer and analyzes the altered DNA signatures of cancerous and precancerous cells that exfoliate into the colon. In addition, this test includes an immunochemical assay for human hemoglobin. The stool samples can be easily collected, mailed from home, and requires no bowel preparation, medication restrictions or dietary change. The purpose of this study was to determine the sensitivity of COLOGUARD® as compared with FIT, for the detection of screening-relevant colorectal cancer. The study was conducted at 90 medical centers throughout the United States and Canada and evaluable patients (N=9989) were asymptomatic individuals between ages 50 and 84 years who were required to provide a stool sample and undergo screening colonoscopy within 90 days. Enrollment was weighted toward those aged 65 years or older. The primary outcome was the ability of COLOGUARD® to detect colorectal cancer and secondary outcomes were the ability of COLOGUARD® to detect advanced precancerous lesions, polyps with high grade dysplasia and serrated sessile polyps measuring 1 cm or more, when compared to FIT. (Sensitivity is defined as the proportion of persons with disease who have a positive test and Specificity is defined as proportion of persons without disease who have a negative test.) The sensitivity for detecting colorectal cancer was 92% with COLOGUARD® and 74% with FIT (P=0.002). The sensitivity for detecting advanced precancerous lesions was 42.4% vs 23.8% (P<0.001), polyps with high-grade dysplasia was 69.2% vs 46.2% (P=0.004) and serrated sessile polyps measuring 1 cm or more were 42.4% vs 5.1%, (P<0.001) with COLOGUARD® and FIT respectively. Specificities with COLOGUARD® were lower compared to FIT (P<0.001). The authors concluded that COLOGUARD® can detect significantly more colorectal cancers and precancerous lesions than FIT. The high sensitivity of this non-invasive, stool DNA test, to detect curable stage of colorectal cancer, may increase the number of people, who will choose to be screened for colorectal cancer. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. N Engl J Med 2014; 370:1287-1297

