SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 246,660 new cases of invasive breast cancer will be diagnosed in 2016 and 40,450 women will die of the disease. Estrogen Receptor (ER) positive breast cancer cells are driven by estrogens. Approximately 60-65% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients.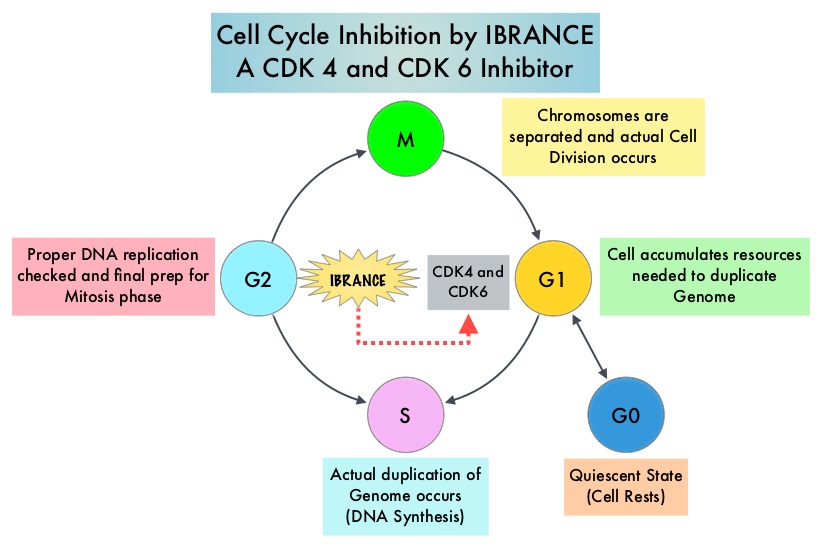
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6), phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein cancels it beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors. IBRANCE® (Palbociclib) is a reversible, oral, selective, small molecule inhibitor of Cyclin Dependent Kinases, CDK4 and CDK6, and prevents RB1 phosphorylation. IBRANCE® is the first CDK inhibitor approved by the FDA. It exhibits synergy when combined with endocrine therapies. The FDA in February 2016, approved IBRANCE® in combination with FASLODEX® (Fulvestrant), for the treatment of women with Hormone Receptor (HR)-positive, Human Epidermal growth factor Receptor 2 (HER2) negative advanced or metastatic breast cancer, with disease progression following endocrine therapy. In a phase II study, a combination of IBRANCE® plus FEMARA® showed improved Progression Free Survival compared with FEMARA® alone, in the initial treatment of postmenopausal women with Estrogen-Receptor (ER) positive, HER2 negative advanced breast cancer. Based on this encouraging data, a phase III study was conducted, to confirm the efficacy and safety of IBRANCE® plus FEMARA® for this patient group.
In this double blind study (PALOMA-2), 666 postmenopausal women with ER positive, HER2 negative breast cancer, who had no prior therapy for advanced disease, were randomly assigned, in a 2:1 ratio to receive IBRANCE® plus FEMARA® (N=444) or placebo plus FEMARA® (N=222). IBRANCE® was administered at 125 mg PO daily, 3 weeks on and 1 week off, every 4 weeks and all patients received FEMARA® 2.5 mg PO daily . The median age was 62 years, 48% had visceral disease and 63% had prior systemic therapy for breast cancer. The primary end point was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate, Clinical Benefit Response and safety.
The median PFS was 24.8 months in the IBRANCE® plus FEMARA® group compared with 14.5 months in the placebo plus FEMARA® group (HR=0.58; P<0.001). This benefit was seen across all patient subgroups. The Objective Response Rate was 42% with the IBRANCE® plus FEMARA® combination and 35% with the placebo plus FEMARA® combination. The Clinical Benefit Response (complete response, partial response or stable disease for 24 weeks or more) was 84% in the IBRANCE® plus FEMARA® group and 71% in the placebo plus FEMARA® group (P<0.001). The most common grade 3 or 4 adverse events were neutropenia noted in 66% of the patients in the IBRANCE® group versus 1.4% in the placebo plus FEMARA® group. Approximately 10% of the patients in the IBRANCE® group permanently discontinued study treatment due to toxicities and 6% did so in the placebo plus FEMARA® group.
The authors concluded that a combination of IBRANCE® and FEMARA® significantly prolonged Progression Free Survival compared with FEMARA® alone, among patients with previously untreated ER-positive, HER2 negative advanced breast cancer and this combination should be the new standard of care for this patient group. Palbociclib and Letrozole in Advanced Breast Cancer. Finn RS, Martin M, Rugo HS, et al. N Engl J Med 2016; 375:1925-1936

