SUMMARY: The FDA on February 7, 2018, approved ZYTIGA® (Abiraterone acetate) in combination with Prednisone for metastatic high-risk Castration Sensitive Prostate Cancer (CSPC). The FDA initially approved ZYTIGA® with prednisone in 2011 for patients with metastatic Castration Resistant Prostate Cancer (CRPC), who had received prior chemotherapy, and the FDA expanded the indication in 2012, for patients with chemo naïve metastatic CRPC. Prostate cancer is the most common cancer in American men with the exclusion of skin cancer and 1 in 9 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 164,690 new cases of Prostate cancer will be diagnosed in 2018 and 29,430 men will die of the disease. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for Castration Sensitive Prostate Cancer (CSPC). Androgen Deprivation Therapies have included bilateral orchiectomy or Gonadotropin Releasing Hormone (GnRH) analogues, with or without first generation androgen receptor inhibitors such as CASODEX®: (Bicalutamide), NILANDRON® (Nilutamide) and EULEXIN® (Flutamide). The median duration of response is approximately 1 year and majority of these patients progress to Castration Resistant Prostate Cancer (CRPC). The mechanism of resistance to Androgen Deprivation Therapy (ADT) include reactivation of Androgen Receptor signaling through persistent adrenal androgen production, modification of the biologic characteristics of Androgen Receptors, intratumoral testosterone production and parallel steroidogenic pathways.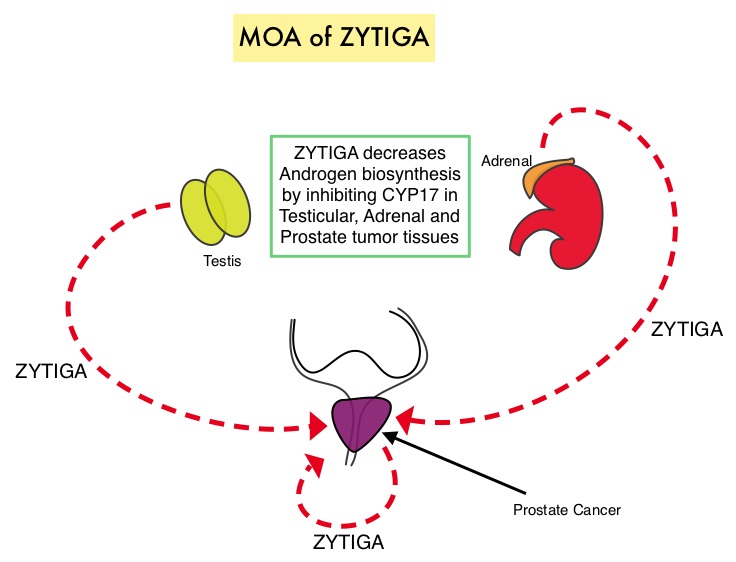
Two previously published trials, STAMPEDE and CHAARTED have shown that the addition of TAXOTERE® (Docetaxel) to Androgen Deprivaton Therapy as first line systemic therapy, significantly improved Overall Survival, among men with locally advanced or metastatic Castration Sensitive Prostate Cancer. This is presently the standard of care for appropriate patients with prostate cancer, who had not received prior hormone therapy. The barriers to chemo-hormonal therapy with TAXOTERE® include advanced patient age, poor Performance Status, comorbidities, patient preferences, as well as potential life threatening toxicities associated with TAXOTERE®.
ZYTIGA® is a selective, irreversible inhibitor of CYP 17A1 enzyme and decreases androgen biosynthesis in the testes, adrenal glands, and prostate-tumor tissue. Combining a CYP17A1 inhibitor such as ZYTIGA® with Androgen Deprivation Therapy is a more effective way of androgen depletion than with Orchiectomy or GnRH analogues alone. ZYTIGA® in combination with prednisone has been shown to significantly increase Overall Survival in patients with metastatic CRPC who had not received chemotherapy as well as those who had received previous chemotherapy with TAXOTERE®. Further, ZYTIGA® along with Prednisone has been shown to reduce tumor burden in men with high-risk, localized prostate cancer, receiving neoadjuvant therapy.
The current FDA approval was based on LATITUDE, a multinational, randomized, double-blind, placebo-controlled, phase III trial, in which the authors evaluated the clinical benefit of adding ZYTIGA® along with Prednisone to Androgen Deprivation Therapy (ADT), as compared with Androgen Deprivation Therapy and placebo, in patients with newly diagnosed, metastatic Castration Sensitive Prostate Cancer. In this study, 1199 newly diagnosed patients with high-risk metastatic prostate cancer were randomized to receive either ZYTIGA® along with Prednisone and ADT (N=597) or placebo and ADT (N=602). ZYTIGA® was administered at 1000 mg and Prednisone at 5 mg, both drugs given orally daily, and ADT consisted of a GnRH (Gonadotropin Releasing Hormone) analog. Eligible patients should not have received prior ADT and had at least 2 of 3 risk factors which included Gleason score 8 or greater, measurable visceral metastases or 3 or more bone lesions, all of which are associated with poor survival. The median age was 68 years and 98% of the enrolled patients had a Gleason score of 8 or more and had 3 or more sites of bone metastases. Both treatment groups were well balanced. The two Primary end points were Overall Survival and radiographic Progression Free Survival.
After a median follow-up of 30.4 months, at a planned interim analysis, the median Overall Survival was significantly longer in the ZYTIGA® group compared to the placebo group (Not Reached versus 34.7 months, HR=0.62; P<0.001). This meant a 38% reduction in the risk of death with the addition of ZYTIGA® and Prednisone to ADT compared with placebo and ADT. The median radiographic Progression Free Survival was 33 months in the ZYTIGA® group and 14.8 months in the placebo group (HR=0.47; P<0.001). This meant a 53% reduction in the risk of progression or death, with the addition of ZYTIGA® and Prednisone to ADT, compared with placebo and ADT. Further in the ZYTIGA® group, significantly better outcomes were observed in all Secondary end points and they included Time to pain progression, Time to next subsequent therapy for prostate cancer, Time to initiation of chemotherapy, and PSA progression (P<0.001 for all comparisons), along with Time to next symptomatic skeletal events (P=0.009). Based on these promising findings, the Independent Data and Safety Monitoring Committee recommended that the trial be unblinded and crossover be allowed for patients in the placebo group to receive ZYTIGA® and Prednisone along with ADT.
The authors concluded that the addition of ZYTIGA® along with Prednisone, to Androgen Deprivation Therapy, significantly increased Overall Survival and radiographic Progression Free Survival, in men with newly diagnosed, metastatic, Castration Sensitive Prostate Cancer. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. Fizazi K, Tran N, Fein L, et al. for the LATITUDE Investigators. N Engl J Med 2017; 377:352-360

