SUMMARY: The FDA on October 24, 2016 approved KEYTRUDA® (Pembrolizumab) for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors have high PD-L1 expression (Tumor Proportion Score greater than or equal to 50%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2016 about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer accounts for approximately 85% of all lung cancers.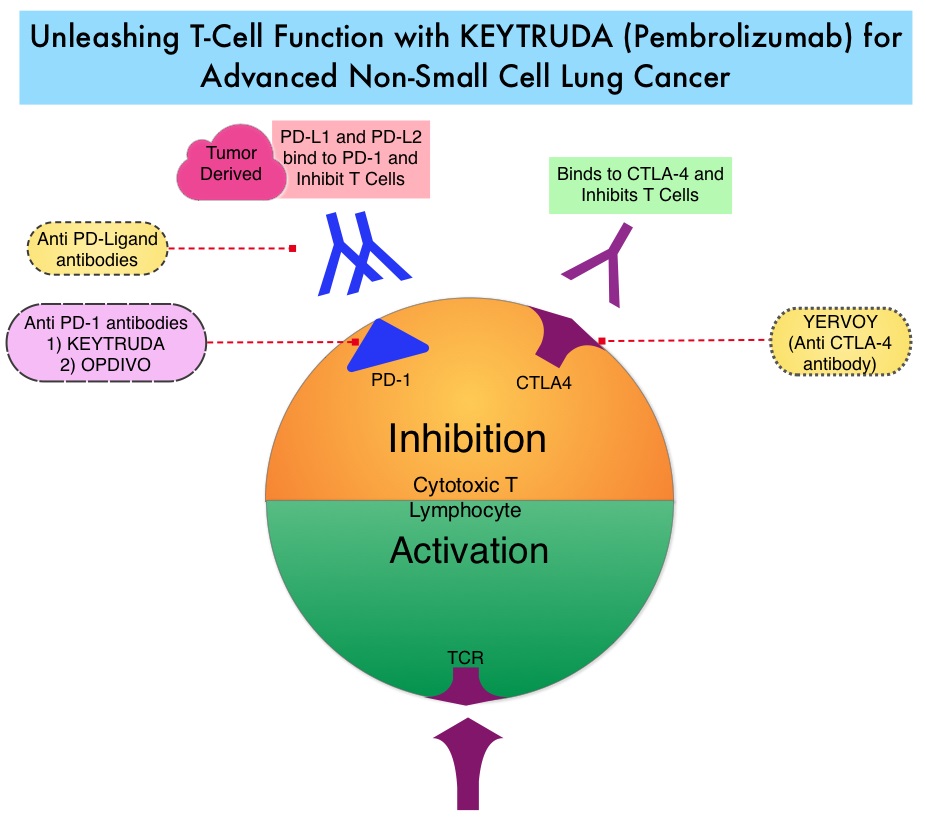
KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced Non Small Cell Lung Cancer (NSCLC) have a high level of PD-L1 expression and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®.
KEYNOTE-024 is a open-label, randomized, phase III trial in which KEYTRUDA® administered at a fixed dose was compared with investigator’s choice of cytotoxic chemotherapy, as first line therapy, for patients with advanced NSCLC, with tumor PD-L1 expression of 50% or greater. Three hundred and five (N=305) treatment naïve patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells, were randomly assigned in a 1:1 ratio to receive either KEYTRUDA® (N=154) or chemotherapy (N=151). Enrolled patients had no sensitizing EGFR mutations or ALK translocations. Treatment consisted of KEYTRUDA® administered at a fixed dose of 200 mg IV every 3 weeks for 35 cycles or the investigator’s choice of platinum-based chemotherapy for 4-6 cycles. Pemetrexed (ALIMTA®) based therapy was permitted only for patients who had non-squamous tumors and these patients could receive ALIMTA® maintenance therapy after the completion of combination chemotherapy. The primary end point was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety.
The median PFS was 10.3 months in the KEYTRUDA® group versus 6.0 months in the chemotherapy group (HR=0.50; P<0.001). This benefit was observed across all patient subgroups including tumor histologic type and chemotherapy regimen administered. The estimated Overall Survival at 6 months was 80.2% in the KEYTRUDA® group versus 72.4% in the chemotherapy group (HR=0.60; P=0.005). Patients in the KEYTRUDA® group experienced higher Response Rates than in the chemotherapy group (44.8% vs. 27.8%) as well as longer median duration of response (Not Reached versus 6.3 months). These benefits were realized even after 43.7% of the patients in the chemotherapy group following progression, had crossed over to receive KEYTRUDA®. Adverse events of any grade were less frequent in the KEYTRUDA® group compared to the chemotherapy group, with diarrhea, fatigue and pyrexia being more common in the KEYTRUDA® group whereas anemia, nausea and fatigue were more often noted in the chemotherapy group. As expected, immune-mediated adverse events (including pneumonitis) occurred more frequently with KEYTRUDA® whereas cytopenias occurred more frequently with chemotherapy.
It was concluded that in treatment naïve patients with advanced NSCLC and a PD-L1 tumor proportion score of 50% or greater, KEYTRUDA® was associated with significantly longer Progression Free and Overall Survival and with fewer adverse events, compared with platinum-based chemotherapy. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. Reck M, Rodríguez-Abreu D, Robinson AG, et al. for the KEYNOTE-024 Investigators. October 9, 2016DOI: 10.1056/NEJMoa1606774

