SUMMARY: The FDA on November 21, 2019, approved CALQUENCE® (Acalabrutinib), for adults with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). The American Cancer Society estimates that for 2019, about 20,720 new cases of Chronic Lymphocytic Leukemia (CLL) will be diagnosed in the US and 3,930 patients will die of the disease. CLL accounts for about 25% of the new cases of leukemia and the average age at the time of diagnosis is around 71 years. B-cell CLL is the most common type of leukemia in adults, accounting for about 11% of all hematologic malignancies.
Bruton's Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. CALQUENCE® is a highly selective, oral, covalent irreversible Bruton Tyrosine Kinase (BTK) inhibitor with minimal activity against other kinases. CALQUENCE® inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. The present FDA approval was based on two randomized, actively controlled trials, ELEVATE-TN and ASCEND in patients with CLL.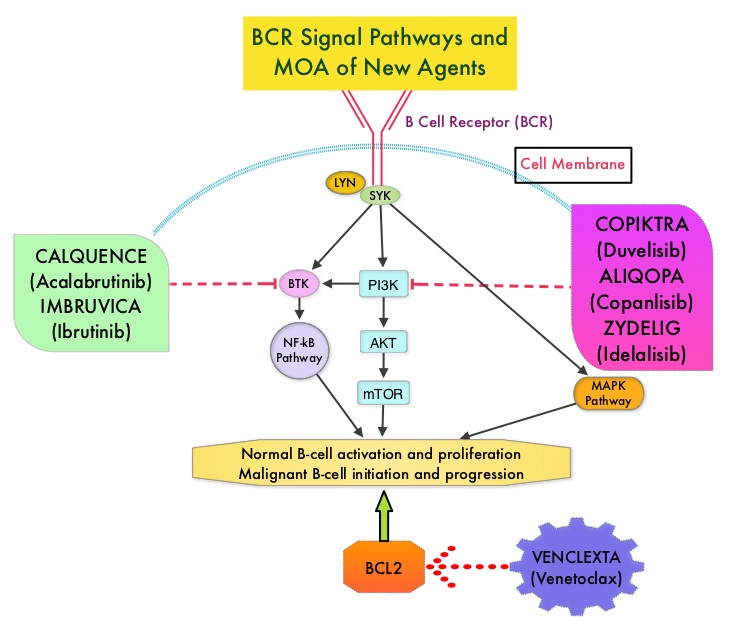
ELEVATE-TN is a randomized, multicentre, open-label Phase III trial which evaluated the safety and efficacy of CALQUENCE® alone or in combination with GAZYVA® (Obinutuzumab) versus Chlorambucil in combination with GAZYVA®, in previously-untreated patients with CLL. GAZYVA® is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody. In this trial, 535 patients were randomized 1:1:1 to receive CALQUENCE® monotherapy 100 mg twice daily orally continuously (N=179) or in combination with GAZYVA® (N=179), or Chlorambucil 0.5 mg/kg on Days 1 and 15 of each 28-day cycle for 6 cycles along with GAZYVA® (N=177). The dose of GAZYVA® was 100 mg on day 1 and 900 mg on day 2 of Cycle 1, 1000 mg on day 8 and 15 of Cycle 1, and 1000 mg on day 1 of Cycles 2–6, of each 28 day cycle. Pts were stratified by del(17p) status, ECOG status (1 or less versus 2), and geographic region. The median patient age was 70 years and 69% had high risk and 12% had very high risk CLL IPI scores. The Primary endpoint was PFS in the CALQUENCE® plus GAZYVA® group compared to the Chlorambucil and GAZYVA® group, assessed by an independent review committee (IRC). A key secondary endpoint was PFS in the CALQUENCE® monotherapy arm, compared to the chlorambucil and GAZYVA® arm. Other secondary endpoints included Objective Response Rate, time to next treatment and Overall Survival.
At a median follow-up of 28 months, the combination of CALQUENCE® plus GAZYVA® significantly prolonged PFS compared to Chlorambucil plus GAZYVA® (median Not Reached versus 22.6 months; HR 0.10, P<0.0001), reducing the risk of progression or death by 90%. CALQUENCE® monotherapy also significantly prolonged PFS compared to Chlorambucil plus GAZYVA® (HR=0.20; P<0.0001). PFS improvement with CALQUENCE® plus GAZYVA® as well as CALQUENCE® monotherapy was consistent across all subgroups examined including del(17p). The median OS was not reached in any treatment group. The ORR was higher with CALQUENCE® plus GAZYVA® versus Chlorambucil plus GAZYVA® (94% versus 79%; P<0.0001). The ORR with CALQUENCE® monotherapy was 85%. Complete Response rates were higher with CALQUENCE® plus GAZYVA® versus Chlorambucil plus GAZYVA® (13% versus 5%).
ASCEND is a global, randomized, multicentre, open-label Phase III trial which evaluated the efficacy and safety of CALQUENCE® versus physician’s choice of Rituximab/ZYDELIG® (Idelalisib) or Rituximab/Bendamustine combination in patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). This study included 310 eligible patients who were randomly assigned 1:1 to receive CALQUENCE® 100 mg orally twice daily until disease progression versus Rituximab/ ZYDELIG® combination (ZYDELIG® 150 mg orally twice daily in combination with up to eight IV infusions of Rituximab 375 or 500 mg/m2) or Rituximab/Bendamustine combination (Bendamustine 70 mg/m2 IV on day 1 and 2 of each cycle in combination with Rituximab 375 or 500 mg/m2 IV on day 1 of each 28-day cycle for up to six cycles). The median age was 67 years and patients were stratified by del(17p) status, ECOG performance scale and prior lines of therapy. The Primary endpoint was Progression Free Survival as assessed by Independent Review Committee (IRC) and Secondary end points included Overall Response Rate and Duration of Response, as well as Overall Survival. Patients with confirmed disease progression on Rituximab/ ZYDELIG® or Rituximab/Bendamustine, were allowed to cross over to receive CALQUENCE®.
At a median follow-up of 16.1 months, there was a statistically significant and clinically meaningful improvement in PFS with CALQUENCE® monotherapy compared to a combination regimen of Rituximab plus physician’s choice of ZYDELIG® or Bendamustine. The PFS with CALQUENCE® monotherapy was not reached versus 16.5 months with the comparators (HR= 0.31, P<0.0001). This represented a 69% reduction in risk of progression or death. Progression Free Survival was improved with CALQUENCE® monotherapy across subgroups including del(17p), TP53 mutation, and Rai stage. There was however no significant difference in the Overall Response Rates among the treatment groups and 23% of the patients randomly assigned to Rituximab/ ZYDELIG® or Rituximab/Bendamustine combinations crossed over to receive subsequent treatment with CALQUENCE®. The most common side effects of CALQUENCE® were anemia, neutropenia, upper respiratory tract infection, thrombocytopenia, headache, diarrhea, and musculoskeletal pain.
In conclusion, CALQUENCE® monotherapy as well as in combination with GAZYVA®, significantly improved Progression Free Survival in Relapsed/Refractory CLL, as well as treatment naïve patients with CLL, respectively.
ELEVATE TN: Phase 3 Study of Acalabrutinib Combined with Obinutuzumab (O) or Alone Vs O Plus Chlorambucil (Clb) in Patients (Pts) with Treatment-Naive Chronic Lymphocytic Leukemia (CLL). Sharman JP, Banerji V, Fogliatto LM, et al. Blood. 2019 Nov 13;134(Supplement_1):31.
ASCEND phase 3 study of acalabrutinib vs investigator’s choice of rituximab plus idelasib (IDR) or bendamustine (BR) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Ghia P, Pluta A, Wach M, et al. Presented at: 2019 European Hematology Association Congress; June 13-16, 2019; Amsterdam, Netherlands. Abstract LB2606.

