SUMMARY: The FDA on February 6, 2019, approved CABLIVI® (Caplacizumab-yhdp) for adult patients with acquired Thrombotic Thrombocytopenic Purpura (aTTP), in combination with Plasma Exchange and immunosuppressive therapy. Thrombotic Thrombocytopenic Purpura (TTP), Hemolytic Uremic Syndrome (HUS) and Atypical Hemolytic Uremic Syndrome (aHUS) are life-threatening Thrombotic MicroAngiopathies (TMAs) associated with systemic microvascular thrombosis, MicroAngiopathic Hemolytic Anemia (MAHA), thrombocytopenia and organ failure. Even though their clinical presentation has some similarities, they are distinct entities with different pathophysiology and hence managed differently.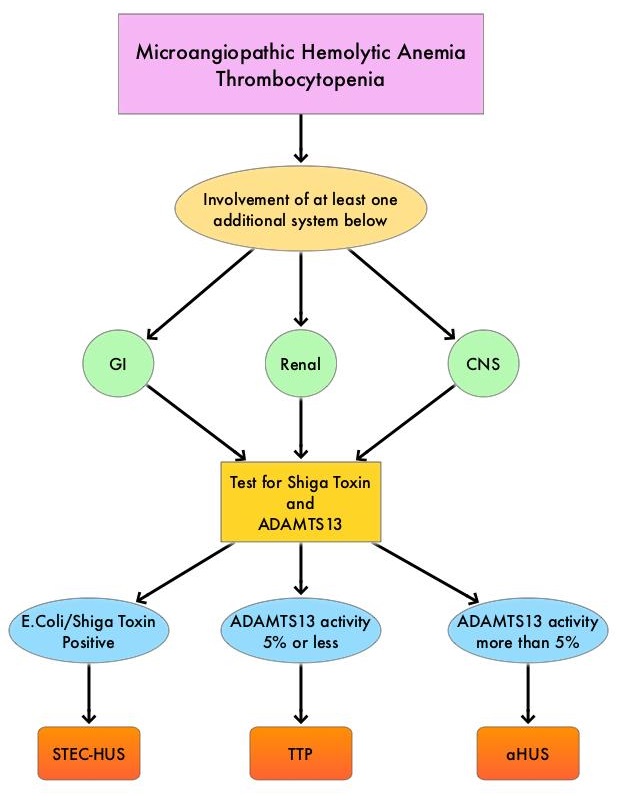
Patients with TTP have either severe deficiency of ADAMTS13 (A Disintegrin And Metalloproteinase with a ThromboSpondin type 1 motif, member 13) secondary to anti-ADAMTS13 autoantibodies or rarely due to ADAMTS13 gene mutations. A diagnosis of TTP is therefore based on clinical presentation and laboratory results and is confirmed by documentation of severe ADAMTS13 deficiency, with ADAMTS13 activity levels of less than 10%. The physiological role of ADAMTS13 is to prevent intravascular platelet thrombosis. ADAMTS13 accomplishes this by cleaving ultralarge von Willebrand Factor multimers (which bind to platelets and induce aggregation), thereby mitigating the tendency of von Willebrand Factor (vWF) and platelets to form aggregates in normal microcirculation. Deficiency of ADAMTS13 causes vWF-platelet aggregation in the arterioles and capillaries, characteristic of TTP, resulting in tissue ischemia from microthrombi and end organ damage. Thus the microthrombi in TTP are platelets bound to vWF and not to fibrinogen. Rapid initiation of Plasma Exchange, the frontline therapy in patients with TTP, removes the anti-ADAMTS13 autoantibodies and ultra large vWF multimers and replenishes ADAMTS13. Immunosuppressive therapy with Glucocorticoids and RITUXAN® (Rituximab) inhibits anti-ADAMTS13 autoantibody formation, by targeting the B lymphocytes. These interventions have significantly improved the survival rate among patients with TTP. Despite these advances with the use of Plasma Exchange, approximately 20% of the patients with TTP will succumb to their disease. There is a persistent risk of life-threatening recurrences, which can occur as late as 10 or 20 years after an initial episode of TTP. Persistent or recurrent ADAMTS13 deficiency is a strong risk factor for recurrence.
CABLIVI® is an anti-von Willebrand Factor, humanized, bivalent, variable-domain-only immunoglobulin fragment (Nanobody), directed against the A1 domain of von Willebrand Factor and prevents the interaction of vWF with the platelet glycoprotein Ib-IX-V receptor and thus prevents microvascular thrombosis.
In a phase II study (TITAN trial), CABLIVI® given as an adjunct to Plasma Exchange significantly reduced the median time to a response, with more rapid normalization of platelet count, LDH and creatinine, and also reduced the incidence of major thromboembolic events as well as mean number of Plasma Exchange days, compared with placebo. However, patients who had persistent ADAMTS13 deficiency had a relapse soon after treatment with CABLIVI® was stopped, suggesting that monitoring of ADAMTS13 could be useful, to guide the continuation of therapy.
HERCULES trial is a multicenter, randomized, double blind, placebo controlled, phase III trial which evaluated the efficacy and safety of CABLIVI® in patients with acquired Thrombotic Thrombocytopenic Purpura. The trial also evaluated the benefit of CABLIVI® in reducing the risk of recurrence, by allowing for treatment to continue until immunosuppressive therapy resolved the underlying autoimmune disease. TTP was diagnosed based on clinical presentation (the presence of both thrombocytopenia and MicroAngiopathic Hemolytic Anemia with schistocytes seen on blood smear) and if they had received exactly one Plasma Exchange treatment. Severe ADAMTS13 deficiency was not an eligibility requirement and patients were excluded if they had suspected thrombotic microangiopathies that were not associated with TTP, such as Hemolytic Uremic Syndrome, or if they had congenital TTP.
This study randomly assigned 145 patients with TTP to receive CABLIVI® (N=72) or placebo (N=73). Patients in both groups received daily Plasma Exchange at 1 to 1.5 times the estimated plasma volume, until at least 2 days after normalization of the platelet count and Glucocorticoids (Prednisone or Prednisolone at a dose of 1 mg/kg or more of body weight per day during the daily Plasma Exchange period and continuing for the first week after the end of the daily Plasma Exchange period. Patients received CABLIVI® 10 mg IV loading bolus or placebo before the start of the first Plasma Exchange after randomization followed by 10 mg daily subcutaneously until 30 days after the last daily Plasma Exchange. Treatment was extended at 7-day intervals for a maximum of 28 days, if signs of persistent underlying disease such as suppressed ADAMTS13 activity levels were still present. The Primary outcome was the time to normalization of the platelet count, with discontinuation of daily Plasma Exchange within 5 days thereafter. Key Secondary outcomes included a composite of TTP-related death, recurrence of TTP, or a thromboembolic event during the trial treatment period, refractory TTP and normalization of organ-damage markers.
It was noted that the median time to normalization of the platelet count was shorter with CABLIVI® than with placebo and patients who received CABLIVI® were 1.55 times as likely to have a normalization of the platelet count as those who received placebo (P=0.01). The composite outcome event was 74% lower with CABLIVI® than with placebo (12% versus 49%, P<0.001). The percentage of patients who had a recurrence of TTP at any time during the trial was 67% lower with CABLIVI® than with placebo (12% versus 38%, P<0.001). None of the patients in the CABLIVI® group developed refractory disease whereas 3 three patients in the placebo group did. Patients who received CABLIVI® required fewer Plasma Exchanges and had a shorter hospital stay, than those who received placebo. The most common adverse reactions in at least 15% of patients receiving CABLIVI® were epistaxis, headache, and gingival bleeding.
It was concluded that among patients with TTP, treatment with CABLIVI® resulted in a more rapid normalization of the platelet count, a lower incidence of a composite of TTP-related death, a lower incidence of recurrence of TTP or a thromboembolic event during the treatment period, and a lower rate of recurrence of TTP during the trial period, compared to placebo. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. Scully M, Cataland SR, Peyvandi F, et al., for the HERCULES Investigators. N Engl J Med 2019; 380:335-346.

