SUMMARY: Prostate Cancer is the most common cancer in American men and approximately 233,000 new cases were diagnosed in 2014 and close to 30,000 men died of the disease. The primary systemic intervention for patients with advanced prostate cancer is Androgen Deprivation Therapy (ADT). This can be accomplished by either surgical castration (bilateral orchicetomy) or medical castration, using LHRH (GnRH- Gonadotropin-Releasing Hormone) agonists. Majority of these patients will eventually develop progressive disease (Castrate Resistant Prostate cancer – CRPC), due to enhanced autocrine and /or paracrine synthesis of androgens or androgen precursors in the tumor microenvironment. This has lead to the development of novel compounds that decrease androgen synthesis as well as androgen signaling in patients with CRPC.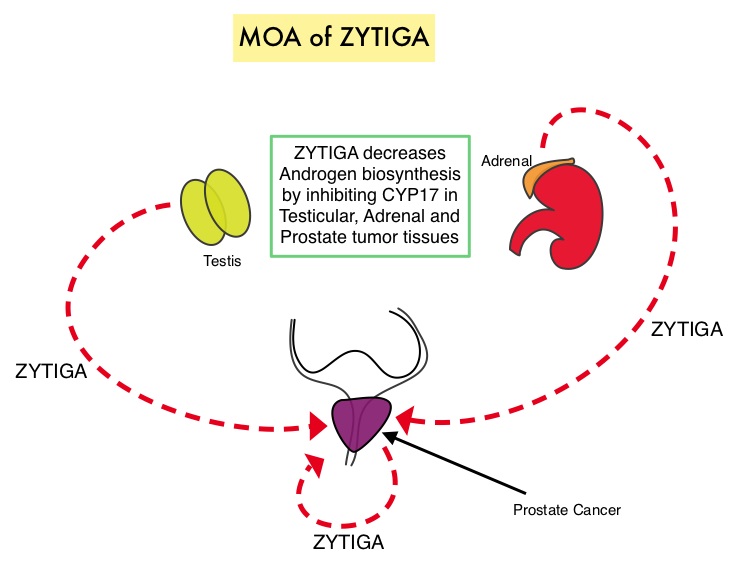 Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. COU-AA-302 trial is a placebo controlled, double-blind, randomized, phase III study and at the interim analyses showed that ZYTIGA® plus Prednisone significantly improved radiographic Progression Free Survival compared with placebo plus Prednisone, in men with chemotherapy-naive CRPC. The authors in this publication provided additional data on the pre-specified final analysis of the trial, which included the effect of ZYTIGA® plus Prednisone on Overall Survival, time to opiate use, and use of other subsequent therapies. In this study which included 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients, 546 patients received ZYTIGA® 1000 mg PO plus Prednisone 5 mg PO twice daily and 542 patients received placebo plus Prednisone. The co-primary endpoints were radiographic Progression Free Survival and Overall Survival. In the final analysis, at a median follow up of 49•2 months, the median Overall Survival was 34.7 months in the ZYTIGA® group vs 30.3 months in the placebo control group (HR = 0.81, P = 0.0033). This benefit was seen across all prespecified subgroups, as well as adjusting for crossover from placebo to ZYTIGA® (HR = 0.79, P = 0.0013). The median time to opiate use for cancer-related pain was significantly prolonged in the ZYTIGA® group compared to the placebo group (33.4 vs 23.4 months, HR = 0.72, P < 0.0001). The most common grade 3 or 4 adverse events of special interest in the ZYTIGA® vs placebo group were cardiac disorders (8% vs 4%), increased ALT (6% vs < 1%), and hypertension (5% vs 3%). The authors concluded that with a median follow up of more than 4 years, treatment with ZYTIGA® in patients with chemotherapy-naive metastatic CRPC, significantly improved Overall Survival compared with Prednisone alone, with favorable toxicities. Ryan CJ, Smith MR, Fizazi K, et al. for the COU-AA-302 Investigators .The Lancet Oncology 2015; 16:152-160
Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. COU-AA-302 trial is a placebo controlled, double-blind, randomized, phase III study and at the interim analyses showed that ZYTIGA® plus Prednisone significantly improved radiographic Progression Free Survival compared with placebo plus Prednisone, in men with chemotherapy-naive CRPC. The authors in this publication provided additional data on the pre-specified final analysis of the trial, which included the effect of ZYTIGA® plus Prednisone on Overall Survival, time to opiate use, and use of other subsequent therapies. In this study which included 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients, 546 patients received ZYTIGA® 1000 mg PO plus Prednisone 5 mg PO twice daily and 542 patients received placebo plus Prednisone. The co-primary endpoints were radiographic Progression Free Survival and Overall Survival. In the final analysis, at a median follow up of 49•2 months, the median Overall Survival was 34.7 months in the ZYTIGA® group vs 30.3 months in the placebo control group (HR = 0.81, P = 0.0033). This benefit was seen across all prespecified subgroups, as well as adjusting for crossover from placebo to ZYTIGA® (HR = 0.79, P = 0.0013). The median time to opiate use for cancer-related pain was significantly prolonged in the ZYTIGA® group compared to the placebo group (33.4 vs 23.4 months, HR = 0.72, P < 0.0001). The most common grade 3 or 4 adverse events of special interest in the ZYTIGA® vs placebo group were cardiac disorders (8% vs 4%), increased ALT (6% vs < 1%), and hypertension (5% vs 3%). The authors concluded that with a median follow up of more than 4 years, treatment with ZYTIGA® in patients with chemotherapy-naive metastatic CRPC, significantly improved Overall Survival compared with Prednisone alone, with favorable toxicities. Ryan CJ, Smith MR, Fizazi K, et al. for the COU-AA-302 Investigators .The Lancet Oncology 2015; 16:152-160

