SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their life time. Approximately, 246,660 new cases of invasive breast cancer will be diagnosed in 2016 and 40,450 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 20-25% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. HERCEPTIN® binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). Adjuvant chemotherapy in combination with HERCEPTIN® has been shown to reduce the relative risk of relapse by 52% and relative risk of death by 33%. ASCO has established a process for adapting clinical practice guidelines of other organizations and this summary of the practice recommendations were adapted from the Cancer Care Ontario evidence based clinical practice guidelines, for the adjuvant treatment of HER2-positive early breast cancers.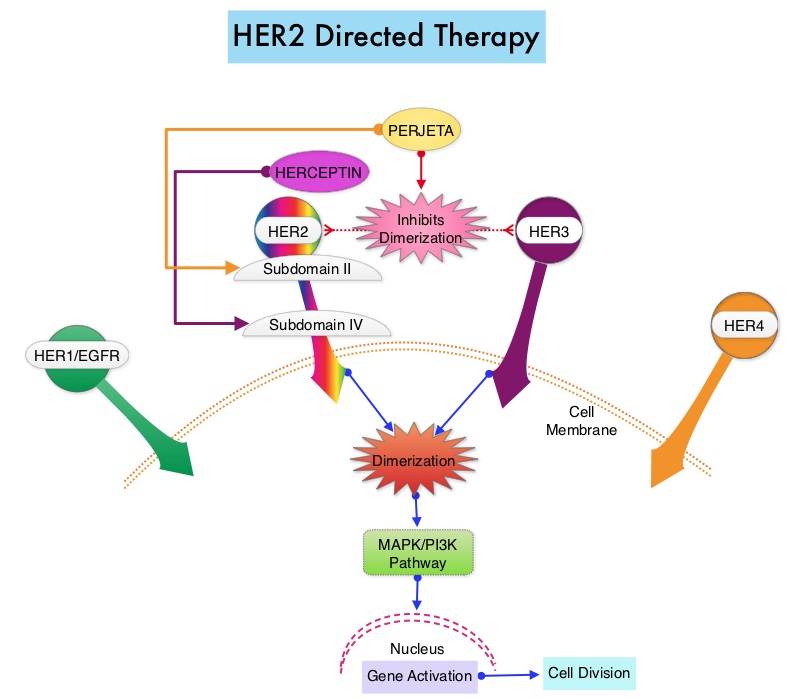
Guideline Question: What is the optimal use of cytotoxic chemotherapy and Human Epidermal growth factor Receptor 2 (HER2) – directed therapy?
Target Population: Female patients who are being considered for, or who are receiving, systemic therapy after definitive surgery for early invasive breast cancer, defined largely as invasive cancer stages I to IIA (T1N0-1, T2N0).
RECOMMENDATIONS
Use of an Anthracycline-Taxane Regimen
1) An adjuvant chemotherapy regimen containing Anthracycline-Taxane is considered the optimal strategy for high risk patients, if they are able to tolerate this regimen.
2) For patients with high-risk disease who will not receive a Taxane, an optimal-dose Anthracycline three-drug regimen (cumulative dose of doxorubicin ≥ 240 mg/m2 or epirubicin ≥ 600 but no higher than 720 mg/m2) that contains Cyclophosphamide, is recommended. The cumulative dose of Doxorubicin in two-drug regimens should not exceed 240 mg/m2.
3) The addition of Gemcitabine or Capecitabine to an Anthracycline-Taxane regimen is not recommended for adjuvant chemotherapy.
Capecitabine in Patients 65 Years of Age and Older
In patients age 65 years or older, Capecitabine is not recommended as an adjuvant chemotherapy option in lieu of standard regimens like Doxorubicin plus Cyclophosphamide (AC) or Cyclophosphamide, Methotrexate, and Fluorouracil (CMF with oral cyclophosphamide).
CMF as an Alternative to AC
For patients in whom Anthracycline-Taxane is contraindicated, CMF (with oral cyclophosphamide) is an acceptable chemotherapy alternative to AC. The ASCO panel recommends classic CMF (oral cyclophosphamide days 1 to 14 with IV Methotrexate-Fluorouracil days 1 and 8, repeated every 28 days for six cycles) as the default adjuvant CMF regimen. However, the panel also recognizes IV CMF regimen given every 21 days.
Acceptable Adjuvant Chemotherapy Regimens for Patients with Higher-Risk Early-Stage Breast Cancer
1) FEC (Fluorouracil, Epirubicin, and Cyclophosphamide) × 3 → T (Docetaxel) × 3 (superior to FEC × 6)
2) AC × 4 → T × 4 (superior to AC × 4)
3) Docetaxel, Doxorubicin, and Cyclophosphamide × 6 (superior to Fluorouracil, Doxorubicin, and Cyclophosphamide × 6)
4) AC × 4 → paclitaxel (P) administered weekly
5) Dose-Dense AC → P (every 2 weeks)
Adjuvant Regimen When an Anthracycline Is Not Preferred
1) Docetaxel plus Cyclophosphamide (TC) × 4 is recommended as an alternative to AC × 4
2) Classic CMF with oral Cyclophosphamide for six cycles. The panel also recognizes IV CMF regimen given every 21 days.
Patient Selection and Adjuvant Trastuzumab Therapy
Only patients with HER2-positive breast cancer (overexpressed based on ImmunoHistoChemistry (IHC 3+) or amplified based on in situ hybridization [ISH ratio ≥ 2.0 or average HER2 copy number ≥ 6.0]), should be offered adjuvant Trastuzumab.
Trastuzumab Plus Chemotherapy
1) Trastuzumab plus chemotherapy is recommended for all patients with HER2-positive, node-positive breast cancer and for patients with HER2-positive, node-negative breast cancer greater than 1 cm in size.
2) Trastuzumab therapy can be considered in small, node-negative tumors (1 cm or less).
3) Trastuzumab can be administered with any acceptable adjuvant chemotherapy regimen.
4) The administration of Trastuzumab concurrently with the Anthracycline component of a chemotherapy regimen is not recommended because of the potential for increased cardiotoxicity.
5) Trastuzumab should be preferentially administered concurrently (not sequentially) with a non-Anthracycline chemotherapy regimen.
6) Less cardiotoxicity is seen with TCH (Docetaxel, Carboplatin, and Trastuzumab) than with AC→TH (Doxorubicin and Cyclophosphamide→Docetaxel and Trastuzumab), and TCH is recommended for patients at higher risk for cardiotoxicity.
7) Even though there is no phase III evidence for the addition of Trastuzumab to some chemotherapy regimens, such as TC, those regimens might be in use and are reasonable options, particularly to mitigate cardiotoxicity in certain patients.
Duration of Trastuzumab Therapy and Cardiac Function Assessment
Patients should be offered 1 year total of adjuvant Trastuzumab, with regular assessments of cardiac function during that period.
Selection of Optimal Adjuvant Chemotherapy Regimens for Early Breast Cancer and Adjuvant Targeted Therapy for HER2-Positive Breast Cancers: An American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. Denduluri N, Somerfield MR, Eisen A, et al. J Clin Oncol 2016;34:2416-2427

