SUMMARY: Prostate cancer is the most common cancer in American men excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 220,800 new cases of prostate cancer will be diagnosed in 2015 and over 27,000 men will die of the disease. Prostate cancer is driven by Androgen Receptor (AR) and its signaling pathways. Initial treatment strategies for patients with metastatic prostate cancer include lowering the levels of circulating androgens with medical or surgical castration or blocking the binding of androgens to the androgen receptor. Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide).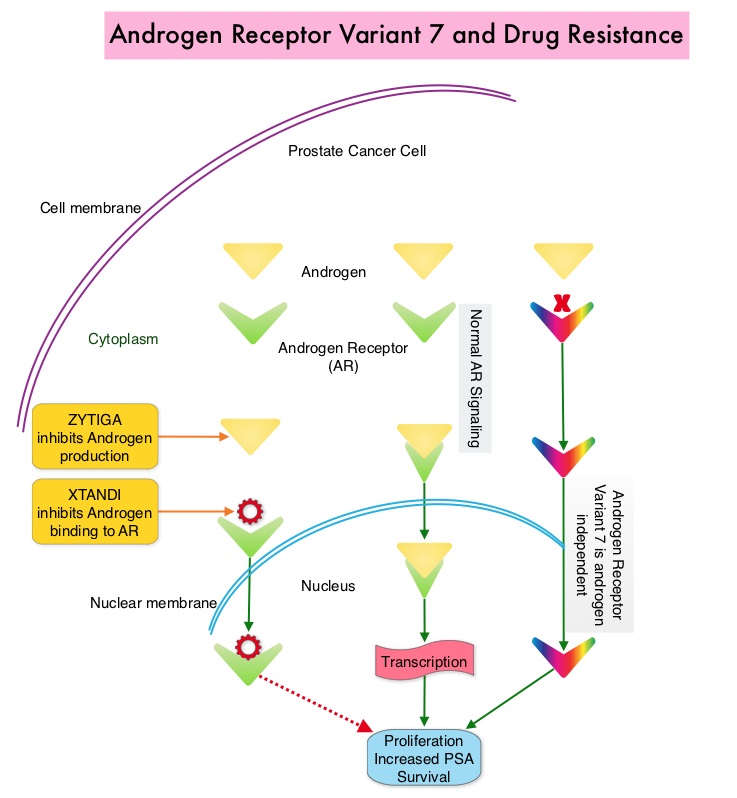 Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)
Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)

