The FDA on November 2, 2018 granted accelerated approval to LORBRENA®, for patients with Anaplastic Lymphoma Kinase (ALK)-positive metastatic Non-Small Cell Lung Cancer (NSCLC) whose disease has progressed on Crizotinib and at least one other ALK inhibitor for metastatic disease or whose disease has progressed on Alectinib or Ceritinib as the first ALK inhibitor therapy for metastatic disease. LORBRENA® is a product of Pfizer, Inc.
Month: November 2018
KEYTRUDA® (Pembrolizumab)
The FDA on October 16, 2018 approved KEYTRUDA® in combination with Carboplatin and either Paclitaxel or nab-Paclitaxel, as first-line treatment of metastatic squamous Non-Small Cell Lung Cancer (NSCLC). KEYTRUDA® is a product of Merck & Co. Inc.
TALZENNA® (Talazoparib)
The FDA on October 16, 2018 approved TALZENNA®, a poly (ADP-ribose) polymerase (PARP) inhibitor, for patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm), HER2 negative, locally advanced or metastatic breast cancer. Patients must be selected for therapy based on an FDA-approved companion diagnostic for TALZENNA®. TALZENNA® is a product of Pfizer Inc.
Testing for BRCA1 and BRCA2 Mutations May Not Be Adequate in Breast Cancer Patients
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that 266,120 new cases of invasive breast cancer will be diagnosed in 2018 and 40,920 women are expected to die from the disease. DNA can be damaged due to errors during its replication or as a result of environmental exposure to ultraviolet radiation from the sun or other toxins. The tumor suppressor genes such as BRCA1 (Breast Cancer 1) and BRCA2 help repair damaged DNA and thus play an important role in maintaining cellular genetic integrity, failing which these genetic aberrations can result in malignancies. The BRCA1 gene is located on the long (q) arm of chromosome 17 whereas BRCA2 is located on the long arm of chromosome 13. Mutations in BRCA1 and BRCA2 account for about 20 to 25 percent of hereditary breast cancers and about 5 to 10 percent of all breast cancers.These mutations can be inherited from either of the parents and a child has a 50 percent chance of inheriting this mutation and the deleterious effects of the mutations are seen even when an individual’s second copy of the gene is normal.
Breast cancer patients have a 5-12% lifetime risk of a second primary cancer. It remains unclear however whether patients with breast cancer and another primary cancer have mutations in genes other than BRCA1 and BRCA2, compared to those with a single breast cancer. There are well established data on the cancer risks, associated with different gene mutations. The authors hypothesized that among these patients, a number of factors including environmental exposures and genetic predisposition, may play a role in the development of more than one primary cancer in their lifetime. Recently published study suggested that there was a 85% cumulative breast cancer risk by age 60 years, among those with mutations in the TP53 gene (Cancer2016;122(23):3673-3681). Further with the increasing recognition that germline mutations in genes may have clinical and treatment implications, majority of patients are feeling comfortable opting for upfront multiple genetic mutation testing.
The researchers in this study looked at a panel of 15 actionable mutations beyond BRCA and the gene panel included TP53, PALB2, CDH1, PTEN, STK11, CHEK2, ATM, NBN, MSH6, PMS2, MSH2, MLH1, CDKN2A, MUTYH monoallelic, and CHEK2 Low Risk. Two cohorts of BRCA1 and BRCA2 negative patients were studied. The first cohort included high-risk breast cancer patients with either a single breast cancer (N=464) or breast cancer and an additional primary cancer (N=551). The second cohort comprised of patients with familial breast cancer (inherited risk) with either a single breast cancer (N=1464) or breast cancer and another primary cancer (N=340).
In a total of 891 patients in both cohorts who had breast cancer and an additional primary cancer, there was twice the risk of inheriting mutations in genes other than BRCA1 and BRCA2. In cohort 1, the mutation rate among patients who had breast cancer and an additional primary cancer was 8.7% compared to 4.1% among those with single breast cancer (P=0.003) and in cohort 2, the mutation rate was 8.2% versus 4.2%, respectively (P=0.003).
There was however a differences in individual gene mutation rates between the two cohorts. Among patients with breast cancer and an additional primary cancer in cohort 1, mutations in TP53 and MSH6 were significantly higher, whereas among the patients in cohort 2 with familial breast cancer, mutations in ATM, CHEK2 and PALB2 were significantly higher both in those with breast cancer and another primary cancer and those with a single breast cancer.
The authors concluded that patients with multiple primary cancers should be offered multiplex panel testing to identify patients at risk. Identifying mutations, especially mutations in the TP53 gene may have a bearing on appropriate recommendations such as risk-reducing bilateral mastectomy or mastectomy instead of a lumpectomy in this patient group. Thus, risk assessment using multiple genetic testing panels can be beneficial for clinical care and surveillance. Inherited mutations in breast cancer patients with and without multiple primary cancers. Maxwell KN, Vijai V, Lilyquist J, et al. DOI: 10.1200/JCO.2018.36.15_suppl.1503 Journal of Clinical Oncology 36, no. 15_suppl (May 2018) 1503-1503.
Radioactive Iodine Increases Risk of Myeloid Malignancies in Patients with Thyroid Cancer
SUMMARY: The American Cancer Society estimates that about 53,990 new cases of thyroid cancer will be diagnosed in the United States for 2018 and about 2,060 patients will die of the disease. Thyroid cancer is the most prevalent endocrine malignancy and is classified into three histological groups – Well Differentiated Thyroid Cancers (94%) which include Papillary (80%), Follicular (11%) and Hürthle cell (3%) histologies, Medullary Thyroid Carcinoma (MTC) representing 4% and Anaplastic (undifferentiated) Thyroid Carcinoma (ATC) representing about 2%. Patients with WDTC often undergo thyroidectomy followed by adjuvant radioactive iodine (RAI) to ablate residual or unresectable disease. Approximately 20% of the patients with WDTC develop local recurrence and 10% develop metastatic disease at 10 years following surgery, RadioIodine Ablation (RIA) and TSH suppressive therapy. There has been a four-fold increase in the incidence of WDTC over the past 30 years and this has been attributed to improved detection of small, low-risk tumors. Majority of these patients are treated with RAI where patients do not derive therapeutic benefit but are rather exposed to its carcinogenic effects. Although prior studies have shown an increased risk of Secondary Hematologic Malignancies in patients with WDTC treated with RAI, these analyses grouped all types of leukemia under one broad category, which oversimplified risk estimation.
The authors conducted this study to investigate the risk and outcomes of second hematologic malignancies (SHMs) among patients with Well Differentiated Thyroid Cancer (WDTC) treated with RadioActive Iodine (RAI). A total of 183,894 patients with thyroid cancer were identified from 18 registries of the National Cancer Institute SEER (Surveillance, Epidemiology, and End Results) program and 148,215 patients with WDTC were included in this study. Among those included, 53% underwent surgery alone, and 47% underwent surgery and received RAI. Patients were excluded if their thyroid malignancy was not of follicular or papillary histology. Low/Intermediate-risk patients with WDTC were defined per the latest American Thyroid Association guidelines as T1-2, N0 tumors 4 cm or less in size, or T1-3, N1 tumors in patients older than 45 years of age.
The Primary outcome was the development of Second Hematologic Malignancies (SHMs), defined as a non-synchronous Hematologic Malignancies occurring 1 year or more after treatment of WDTC. SHMs included in this study were Acute Myeloid Leukemia (AML), Chronic Myeloid Leukemia (CML), Acute Lymphoblastic Leukemia (ALL), Chronic Lymphocytic Leukemia (CLL), Hodgkin Lymphoma, Non-Hodgkin Lymphoma, and Multiple Myeloma (MM). SHMs occurring less than 1 year after WDTC diagnosis were also excluded. The authors performed a competing risk regression analysis to calculate the risks of SHMs that occurred after WDTC treatment and they assessed the outcomes after SHM diagnosis.
At a median follow up of 6.5 years after WDTC diagnosis, 783 patients developed a Secondary Hematologic Malignancy. In multivariate analysis, when compared with those undergoing thyroidectomy alone, RAI treatment was associated with an increased early risk of developing AML (HR=1.79; P=0.01) and CML (HR=3.44; P<0.001). This increased risk of AML and CML after RAI treatment was seen even in low-risk and intermediate-risk WDTC tumors. In those patients with WDTC developing AML, the median Overall Survival was significantly shorter compared with matched controls (8 years versus 31 years; P=0.001). Further, among those developing AML after RAI treatment, median Overall Survival was inferior compared to matched controls with de novo AML (1.2 years versus 2.9 years; P=0.06).
The authors concluded that patients with Well Differentiated Thyroid Cancer (WDTC) treated with RAI are at an increased early risk of developing AML and CML. Patients developing AML following treatment with RAI have a poor prognosis. RAI use in patients with WDTC should therefore be limited to patients with high-risk disease features, and patients with WDTC treated with adjuvant RAI should be monitored for myeloid malignancies as part of cancer surveillance. Risk of Hematologic Malignancies After Radioiodine Treatment of Well-Differentiated Thyroid Cancer. Molenaar RJ, Sidana S, Radivoyevitch T, et al. J Clin Oncol 2017;36:1831-1839
FDA Approves EMPLICITI® Combination for Relapsed Refractory Multiple Myeloma
SUMMARY: The FDA on November 6, 2018 approved EMPLICITI® (Elotuzumab) in combination with POMALYST® (Pomalidomide) and Dexamethasone for the treatment of adult patients with Multiple Myeloma who have received at least two prior therapies, including REVLIMID® (Lenalidomide) and a Proteasome Inhibitor. Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 30,770 new cases will be diagnosed in 2018 and 12,770 patients are expected to die of the disease. Multiple Myeloma (MM) in 2018 remains an incurable disease. The therapeutic goal therefore is to improve Progression Free Survival (PFS) and Overall Survival (OS). Despite the introduction of novel therapies for the treatment of Multiple Myeloma, many patients still face poor outcomes in the Relapsed/Refractory setting.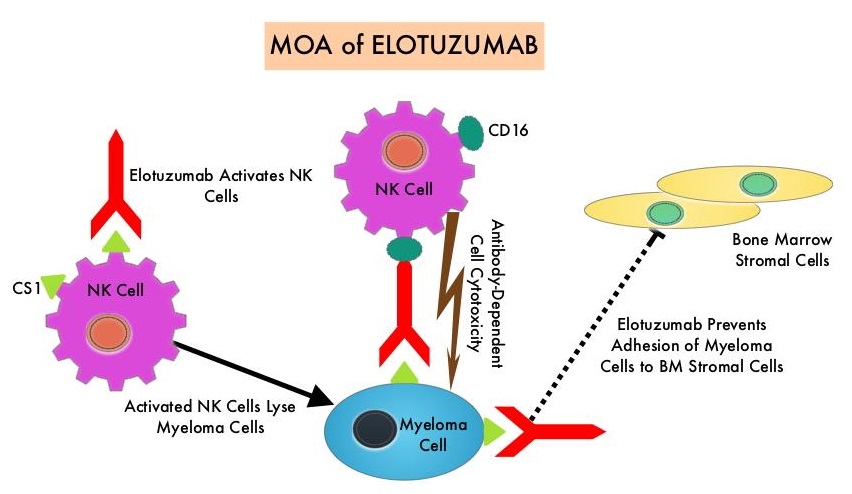
EMPLICITI® is a immunostimulatory monoclonal antibody that binds to the Signal Lymphocyte Activation Molecule – SLAMF7 protein (CS1, CD319), which is highly expressed on Myeloma cells and also expressed on Natural Killer (NK) lymphocytes in the immune system. By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. The FDA in November 2015, approved EMPLICITI® for use in combination with REVLIMID® and Dexamethasone for the treatment of patients with Multiple Myeloma who received one to three prior therapies, based on the ELOQUENT-2 trial.
ELOQUENT-3 is a multicenter, randomized, open label, phase II trial in which 117 patients with Relapsed/Refractory Multiple Myeloma were randomly assigned, in a 1:1 ratio, to receive EMPLICITI® plus POMALYST® and Dexamethasone (N=60) – EMPLICITI® group or POMALYST® and Dexamethasone (N= 57) – control group. Treatment was administered in 28-day cycles. Patients in the EMPLICITI® group received EMPLICITI® 10 mg/kg IV days 1, 8, 15, and 22 during cycles 1 and 2 and 20 mg/kg on day 1 of each cycle thereafter. POMALYST® was given at 4 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly for patients 75 years of age or less or 20 mg for those more than 75 years of age. Treatment was continued until disease progression or unacceptable toxicity. Eligible patients had received 2 or more prior lines of therapy and prophylaxis against thromboembolism was required for all patients. The median age of patients was 67 years. Prior treatments included VELCADE®- Bortezomib (100%), REVLIMID® (99%), KYPROLIS® – Carfilzomib (21%), NINLARO® – Ixazomib (6%), and DARZALEX® – Daratumumab (3%). Close to 55% of patients had undergone Stem Cell transplantation and most patients were refractory to REVLIMID® (87%), a Proteosome Inhibitor (80%), or both (70%). The Primary end point was Progression Free Survival (PFS). The Secondary endpoints included Overall Response Rate (ORR), Complete Response (CR), Stringent Complete Response, Very Good Partial Response and Partial Response Rates.
After a minimum follow up period of 9.1 months, the median PFS was 10.3 months in the EMPLICITI® group and 4.7 months in the control group (HR=0.54; P=0.008), which suggested a 46% reduction in the risk of disease progression. The PFS of EMPLICITI® was consistently observed across all predefined patient subgroups. The Overall Response Rate was 53% in the EMPLICITI® group as compared with 26% in the control group, suggesting a doubling in the Response Rates in the EMPLICITI® group (P=0.0029) with Very Good Partial Responses or better seen in 20% of those in the EMPLICITI® group. The Overall Survival data were immature at the time of the analysis. However, a trend favoring the EMPLICITI® group was observed (HR for death=0.62).
The incidence of serious adverse events was 53% in the EMPLICITI® group and 55% in the control group and the most common treatment-related adverse events in the EMPLICITI® and control groups were neutropenia (18% versus 20%), hyperglycemia (18% versus 11%) and anemia (10% versus 15%), respectively. Adverse events that led to discontinuation of treatment occurred in 18% of the patients in the EMPLICITI® group and in 24% of the patients in the control group.
It was concluded that among patients with Multiple Myeloma who had progressed on REVLIMID® and a Proteosome Inhibitor, EMPLICITI® combined with POMALYST® and Dexamethasone significantly decreased the risk of progression or death and also doubled the Response Rate, compared to POMALYST® plus Dexamethasone alone. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. Dimopoulos MA, Dytfeld D, Grosicki S, et al. N Engl J Med 2018;379:1811-1822
ASCO Endorses Complementary Therapy Guidelines for Breast Cancer Patients
SUMMARY: The American Society for Clinical Oncology (ASCO) after careful review, endorsed the Society for Integrative Oncology (SIO) guideline on the use of integrative therapies during and after breast cancer treatment. Integrative medicine is defined as a patient-centered, evidence-informed field of care that uses mind and body practices, natural products, and/or lifestyle modifications to improve health, quality of life, and clinical outcomes. The ASCO Expert Panel determined that the recommendations in the SIO guideline published in 2017 are clear, thorough, and based on the most relevant scientific evidence. The panel emphasized that these therapies are complementary and should be used along with conventional anticancer treatment, thereby taking a more comprehensive treatment approach across the cancer care continuum, to manage symptoms and adverse effects of breast cancer treatment. This ASCO guideline excluded lifestyle changes such as diet and exercise, mainstream interventions such as support groups, and practices like attention-restoration therapy, that are still being evaluated. Practices such as prayer and spirituality were not considered specifically integrative oncology therapy.
Guideline Question
What are evidence-based approaches to the use of integrative therapies in the management of symptoms and adverse effects during and after breast cancer treatment?
Target Population
Patients undergoing treatment of breast cancer and survivors of breast cancer
Target Audience
Oncologists, integrative medicine providers, supportive care specialists, nurses, pharmacists, primary care providers, and patients with breast cancer
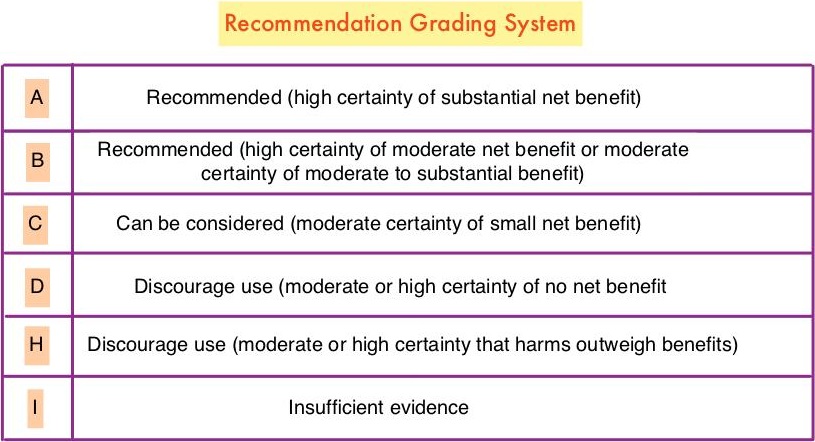
Key Recommendations
Acute Radiation Skin Reaction
Aloe vera and hyaluronic acid cream should not be recommended for improving acute radiation skin reaction. (Grade D)
Anxiety and Stress Reduction
Meditation is recommended for reducing anxiety. (Grade A)
Music therapy is recommended for reducing anxiety. (Grade B)
Stress management is recommended for reducing anxiety during treatment, but longer group programs are likely better than self-administered home programs or shorter programs. (Grade B)
Yoga is recommended for reducing anxiety. (Grade B)
Acupuncture, massage, and relaxation can be considered for reducing anxiety. (Grade C)
Chemotherapy-Induced Nausea and Vomiting
Acupressure can be considered as an addition to antiemetic drugs to control nausea and vomiting during chemotherapy. (Grade B)
Electroacupuncture can be considered as an addition to antiemetic drugs to control vomiting during chemotherapy. (Grade B)
Ginger and relaxation can be considered as additions to antiemetic drugs to control nausea and vomiting during chemotherapy. (Grade C)
Glutamine should not be recommended for improving nausea and vomiting during chemotherapy. (Grade D)
Depression and Mood Disturbance
Meditation, particularly mindfulness-based stress reduction, is recommended for treating mood disturbance and depressive symptoms. (Grade A)
Relaxation is recommended for improving mood disturbance and depressive symptoms. (Grade A)
Yoga is recommended for improving mood disturbance and depressive symptoms. (Grade B)
Massage is recommended for improving mood disturbance. (Grade B)
Music therapy is recommended for improving mood disturbance. (Grade B)
Acupuncture, healing touch, and stress management can be considered for improving mood disturbance and depressive symptoms. (Grade C)
Fatigue
Hypnosis and ginseng can be considered for improving fatigue during treatment. (Grade C)
Acupuncture and yoga can be considered for improving post-treatment fatigue. (Grade C)
Acetyl-l-carnitine and guarana should not be recommended for improving fatigue during treatment. (Grade D)
Lymphedema
Low-level laser therapy, manual lymphatic drainage, and compression bandaging can be considered for improving lymphedema. (Grade C)
Neuropathy
Acetyl-l-carnitine is not recommended for the prevention of chemotherapy-induced peripheral neuropathy in patients with breast cancer due to potential harm. (Grade H)
Pain
Acupuncture, healing touch, hypnosis, and music therapy can be considered for the management of pain. (Grade C)
Quality of Life
Meditation is recommended for improving quality of life. (Grade A)
Yoga is recommended for improving quality of life. (Grade B)
Acupuncture, mistletoe, qigong, reflexology, and stress management can be considered for improving quality of life. (Grade C)
Sleep Disturbance
Gentle yoga can be considered for improving sleep. (Grade C)
Vasomotor/Hot Flashes
Acupuncture can be considered for improving hot flashes. (Grade C)
Soy is not recommended for hot flashes in patients with breast cancer due to lack of effect. (Grade D)
Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. Lyman GH, Greenlee H, Bohlke K, et al. J Clin Oncol. 2018;36:2647-2655
Circulating Tumor DNA (Liquid Biopsy) Can Predict Outcomes in Diffuse Large B-Cell Lymphoma
SUMMARY: The American Cancer Society estimates that in 2018, about 74,680 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 19,910 individuals will die of this disease. Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3-4% each year. More than half of patients are 65 or older at the time of diagnosis and the incidence is likely to increase with the aging of the American population. The etiology of Diffuse Large B-Cell Lymphoma is unknown. Contributing risk factors include immunosuppression (AIDS, transplantation setting, autoimmune diseases), UltraViolet radiation, pesticides, hair dyes, and diet. DLBCL is a neoplasm of large B cells and the most common chromosome abnormality involves alterations of the BCL-6 gene at the 3q27 locus, which is critical for germinal center formation. Two major molecular subtypes of DLBCL arising from different genetic mechanisms have been identified, using gene expression profiling: Germinal Center B-cell-like (GCB) and Activated B-Cell-like (ABC). Patients in the GCB subgroup have a higher five year survival rate, independent of clinical IPI (International Prognostic Index) risk score, whereas patients in the ABC subgroup have a significantly worse outcome. Regardless, R-CHOP regimen (RITUXAN®-Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone), given every 21 days, for 6 cycles, delivered with curative intent, is the current standard of care for patients of all ages, with newly diagnosed DLBCL, regardless of molecular subtype.
Approximately 30-40% of patients experience disease progression or relapse, during the first 2 years and it is important to be able to predict which patients will need more aggressive intervention earlier. Circulating tumor DNA (ctDNA) is being increasingly used as a biomarker across various tumor types and the researchers have previously shown that ctDNA levels can predict tumor recurrence weeks or months before clinical symptoms arise, in patients with lung cancer.
The use of International Prognostic Index (IPI) and interim Positron Emission Tomography (PET) to select patients for intensified therapy, has failed to improve Overall Survival in patients with DLBCL. Several recent studies have suggested that for patients with DLBCL, the addition of pretreatment tumor genomic information to the established clinical prognostic tools such as the International Prognostic Index (IPI) score and interim positron emission tomography (PET) may greatly improve risk stratification. However, the association between the use of this risk stratification to select patients for more aggressive therapy and improvement in outcomes has remained unclear.
The authors in this study evaluated whether dynamic and serial measurements of circulating tumor DNA (ctDNA) from plasma of DLBCL patients had additional prognostic value for predicting patient outcomes, before and during therapy of Diffuse Large B-Cell Lymphoma, as the existing methods are unable to consistently predict treatment failure, given the heterogeneity of DLBCL.
The researchers measured ctDNA from 217 patients with DLBCL or Primary Mediastinal B-cell Lymphoma at six centers in the US and Europe using CAPP-Seq (Cancer Personalized Profiling by deep sequencing ) methodology and compared levels of ctDNA before treatment with levels after the first and second cycles of conventional chemotherapy for each patient. CAPP-Seq is a form of targeted deep next-generation sequencing invented by these authors. They applied deep sequencing of genes for mutational genotyping and tracking of mutations before and during therapy for predicting Event Free Survival (EFS) at 24 months as well as Overall Survival (OS). Using this method, 99% of patients had at least one tumor-specific alteration detected for monitoring, and 98% had ctDNA prior to therapy. Further, the researchers also assessed the prognostic value of ctDNA in the context of established risk factors, including the International Prognostic Index and interim Positron Emission Tomography/Computed Tomography scans.
It was noted that Pretreatment ctDNA levels were prognostic in both the frontline and the relapsed setting. A two-log (i.e., 100-fold) decrease in ctDNA between pretreatment samples and end of cycle 1 of therapy (Early Molecular Response-EMR) or a 2.5-log drop in ctDNA between pretreatment samples and end of cycle 3 of therapy (Major Molecular Response-MMR) was associated with a substantially higher likelihood of cure, independent of the IPI score, in the multivariate analysis. Early Molecular Response and Major Molecular Response were concordant in 92% of patients in whom both were evaluable.
The 24-month Event Free Survival among patients receiving frontline therapy who experienced an Early Molecular Response was 83% vs. 50% (P=0.0015) and for those who experienced a Major Molecular Response was 82% vs. 46% (P<0.001). Early Molecular Response also predicted superior 24-month EFS among patients receiving salvage therapy (100% vs. 13%; P =0.011).
The molecular responses as defined by the drop in ctDNA levels, when correlated with established risk factors such as IPI and interim PET/CT scans, remained prognostic for EFS and OS in patients with low or high IPI, as well as in the context of interim PET/CT. It was noted that patients with favorable results for both molecular response and interim PET had excellent outcomes, whereas those with a combination of a positive interim PET scan and no molecular response were at extremely high risk for treatment failure.
It was concluded that pretreatment ctDNA levels and molecular responses are independently prognostic of outcomes in DLBCL. ctDNA could be used as a risk stratification tool and those who do not have a rapid drop in the ctDNA levels ( within 21 days after the initiation of treatment) may be candidates for more aggressive or novel therapies. More importantly standardization of the available assays for molecular monitoring in DLBCL will be necessary. Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. Kurtz DM, Scherer F, Jin MC, et al. J Clin Oncol. 2018 Oct 1;36(28):2845-2853. doi: 10.1200/JCO.2018.78.5246. Epub 2018 Aug 20.
BRAF V600E Mutation is a Very Poor Prognostic Factor in Metastatic Colorectal Carcinoma
SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 140,250 new cases of CRC will be diagnosed in the United States in 2018 and about 50,630 patients are expected to die of the disease. The lifetime risk of developing CRC is about 1 in 21 (4.7%). Advanced colon cancer is often incurable and standard chemotherapy when combined with anti EGFR (Epidermal Growth Factor Receptor) targeted monoclonal antibodies such as VECTIBIX® (Panitumumab) and ERBITUX® (Cetuximab) as well as anti VEGF agent AVASTIN® (Bevacizumab), have demonstrated improvement in Progression Free Survival (PFS) and Overall Survival (OS). The benefit with anti EGFR agents however is only demonstrable in patients with metastatic CRC, whose tumors do not harbor KRAS mutations in codons 12 and 13 of exon 2 (KRAS Wild Type). It is now also clear that even among the KRAS Wild Type patients, about 15-20% have other rare mutations such as NRAS and BRAF mutations, which confer resistance to anti EGFR agents. Patients with stage IV colorectal cancer are now routinely analyzed for extended RAS and BRAF mutations. KRAS mutations are predictive of resistance to Epidermal Growth Factor Receptor (EGFR) targeted therapy. Approximately 5-10% of all metastatic CRC tumors present with BRAF V600E mutations and BRAF V600E is recognized as a marker of poor prognosis in this patient group. These patients tend to have aggressive disease with a higher rate of peritoneal metastasis and do not respond well to standard treatment intervention. Approximately 25% of the BRAF-mutated population in the metastatic setting has MSI-High tumors, but MSI-High status does not confer protection to this patient group.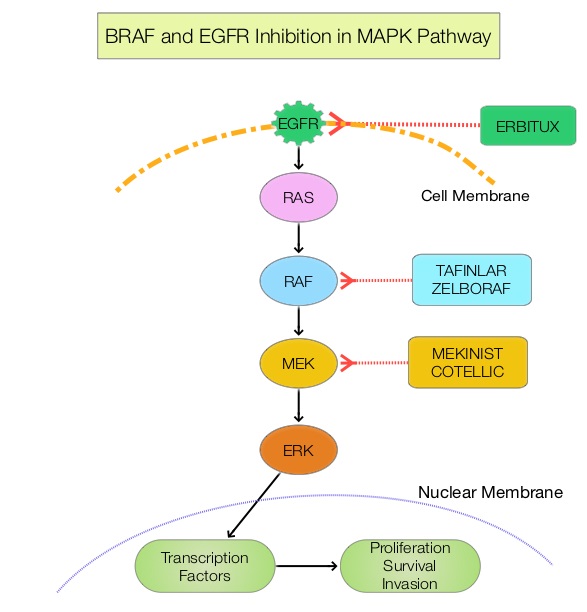
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. The BRAF V600E mutations results in constitutive activation of the MAP kinase pathway. Inhibiting BRAF can transiently reduce MAP kinase signaling. However, this can result in feedback upregulation of EGFR signaling pathway, which can then reactivate the MAP kinase pathway. This aberrant signaling can be blocked by dual inhibition of both BRAF and EGFR.
ZELBORAF® (Vemurafenib), is a selective oral inhibitor of mutated BRAF whereas ERBITUX® is a monoclonal antibody targeting Epidermal Growth Factor Receptor (EGFR). In a phase II SWOG trial (SWOG 1406), the addition of ZELBORAF® to the combination of CAMPTOSAR® (Irinotecan) and ERBITUX® resulted in a 58% reduction in the risk of disease progression and a higher Disease Control Rate, suggesting that simultaneous EGFR and BRAF inhibition (Dual Inhibition) is effective in BRAF V600 mutated ColoRectal Cancer.
Unlike primary colorectal cancer, the association of BRAF V600E and non-V600E mutations with survival and tumor recurrence after resection of ColoRectal Liver Metastases (CRLM), has remained unclear. This present study was conducted to investigate the prognostic association of BRAF mutations with survival and recurrence independently, and to understand how BRAF mutations compared with other prognostic determinants, such as KRAS mutations. This cohort study enrolled 853 patients with colorectal tumors and liver metastases, of whom 849 patients were evaluable and included in the study analyses. All patients underwent resection of their ColoRectal Liver Metastases with a curative intent from January 1, 2000, through December 31, 2016, at institutions participating in the International Genetic Consortium for Colorectal Liver Metastasis and had available data on BRAF and KRAS mutational status. The median age was 60 years, and 60% were male. The main outcomes and measures were the association of BRAF V600E and non-V600E mutations with Disease Free Survival (DFS) and Overall Survival (OS).
Forty three patients (5.1%) had a mutated BRAF (V600E and non-V600E) /wild-type KRAS genotype, 480 patients (56.5%) had wild-type BRAF/wild-type KRAS genotype; and 326 patients (38.4%) had a wild-type BRAF/mutated KRAS genotype. Compared with the wild-type BRAF/wild-type KRAS genotype group, patients with a mutated BRAF/wild-type KRAS genotype more frequently were female (62.8% vs 35.2%) and 65 years or older (51.2% vs 36.9%), had right-sided primary tumors (62.8% vs 17.4%), and presented with a metachronous liver metastasis (64.3% vs 46.8%). The median follow up was 28.3 months.
On multivariable analysis, the presence of BRAF V600E but not non-V600E mutation was associated with significantly poor Overall Survival (HR=2.76; P<0.001) and Disease Free Survival (HR=2.04; P=0.002). Compared with KRAS mutation, the BRAF V600E mutation had a stronger association with OS and DFS than the KRAS mutations.
It was concluded that the presence of BRAF V600E mutation was associated with worse prognosis and increased risk of recurrence, and BRAF V600E mutation was not only a stronger prognostic factor than KRAS but also was the strongest prognostic determinant in the overall cohort. It remains to be seen if BRAF V600E mutated metastatic colorectal tumors would have better outcomes with targeted triplet combination therapies such as ZELBORAF® CAMPTOSAR® and ERBITUX® or TAFINLAR® (Dabrafenib-BRAF inhibitor), MEKINIST® (Trametinib-MEK inhibitor) and VECTIBIX®. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. Margonis, GA, Buettner, S, Andreatos, N, et al. JAMA Surg. 2018;153(7):e180996. doi:10.1001/jamasurg.2018.0996
IMFINZI® after Chemoradiotherapy Significantly Improves Overall Survival in Stage III NSCLC
IMFINZI® (Durvalumab) is a selective, high-affinity, human IgG1 monoclonal antibody, that blocks the binding of Programmed Death Ligand 1 (PD-L1) to Programmed Death 1 (PD-1) receptor and CD80, thereby unleashing the T cells to recognize and kill tumor cells. Patients with stage III Non Small Cell Lung Cancer (NSCLC) are often treated with platinum-based doublet chemotherapy with concurrent radiation and have a median Progression Free Survival (PFS) of approximately 8 months and 5 year survival of only 15%. PACIFIC trial is a randomized, double-blind, international, phase III study in which IMFINZI® as consolidation therapy was compared with placebo, in patients with stage III, locally advanced, unresectable NSCLC, that had not progressed following platinum-based chemoradiotherapy.
At a median follow up of 25.2 months, the 24-month Overall Survival rate was 66.3% in the IMFINZI® group and 55.6% in the placebo group, suggesting a significantly prolonged Overall Survival with IMFINZI® when compared with placebo and a 32% reduction in the risk of death (HR for death=0.68; P=0.0025). The Overall Survival benefit with IMFINZI®, was observed across all the prespecified subgroups.
PACIFIC trial is the first study to demonstrate a survival advantage for unresectable Stage III NSCLC, supporting this regimen as the standard of care.
