The FDA on July 31, 2020 granted accelerated approval to MONJUVI®, a CD19-directed cytolytic antibody, indicated in combination with REVLIMID® (Lenalidomide), for adult patients with Relapsed or Refractory Diffuse Large B-cell Lymphoma (DLBCL) Not Otherwise Specified (NOS), including DLBCL arising from Low grade lymphoma, and who are not eligible for Autologous Stem Cell Transplant. MONJUVI® is a product of MorphoSys US Inc.
Tag: Non-Hodgkin Lymphoma
FDA Approves MONJUVI® for Diffuse Large B-Cell Lymphoma
SUMMARY: The FDA on July 31, 2020, granted accelerated approval to MONJUVI® (Tafasitamab-cxix), a CD19-directed cytolytic antibody, in combination with REVLIMID® (Lenalidomide), for adult patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL) Not Otherwise Specified, including DLBCL arising from low grade lymphoma, and who are not eligible for Autologous Stem Cell Transplant.
The American Cancer Society estimates that in 2020, about 77,240 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 19,940 individuals will die of this disease. Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3-4% each year. More than half of patients are 65 or older at the time of diagnosis and the incidence is likely to increase with the aging of the American population. The etiology of Diffuse Large B-Cell Lymphoma is unknown. Contributing risk factors include immunosuppression (AIDS, transplantation setting, autoimmune diseases), UltraViolet radiation, pesticides, hair dyes, and diet. DLBCL is a neoplasm of large B cells and the most common chromosome abnormality involves alterations of the BCL-6 gene at the 3q27 locus, which is critical for germinal center formation. Two major molecular subtypes of DLBCL arising from different genetic mechanisms have been identified, using Gene Expression Profiling: Germinal Center B-cell-like (GCB) and Activated B-Cell-like (ABC). Patients in the GCB subgroup have a higher five year survival rate, independent of clinical IPI (International Prognostic Index) risk score, whereas patients in the ABC subgroup have a significantly worse outcome. Regardless, R-CHOP regimen (RITUXAN®-Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone), given every 21 days, for 6 cycles, delivered with curative intent, is the current standard of care for patients of all ages, with newly diagnosed DLBCL, regardless of molecular subtype. Approximately 30-40% of patients experience disease progression or relapse, during the first 2 years and attempts to improve on R-CHOP regimen have not been successful. Maintenance treatment strategy following R-CHOP, to better control the disease, delay disease progression and improve long term survival, have included Autologous Stem Cell Transplantation, maintenance treatment with agents such as oral protein kinase inhibitor Enzastaurin and Everolimus. Outcomes for transplant-ineligible patients with Relapsed/Refractory DLBCL patients remain poor.
REVLIMID® (Lenalidomide) is an oral immunomodulatory agent with activity in lymphoid malignancies, primarily through immune modulation (repair T-cell immune synapse dysfunction and Natural Killer cell/T-cell effector augmentation). It additionally has antiproliferative effects. REVLIMID® was shown to have significant activity in relapsed DLBCL when given alone or along with RITUXAN®. MONJUVI® is an investigational humanized Fc-engineered monoclonal antibody directed against CD19. MONJUVI® incorporates an XmAb(R) engineered Fc domain, which is intended to lead to a significant potentiation of Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular Phagocytosis (ADCP), thus improving tumor cell kill. Preclinical data suggested that MONJUVI® might act synergistically with REVLIMID®.
L-MIND is an ongoing , multicenter, single arm, open-label, Phase II study, investigating the combination of MONJUVI® and REVLIMID® in patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL), after up to two prior lines of therapy, including an anti-CD20 targeting therapy (such as Rituximab), who are not eligible for high-dose chemotherapy and subsequent Autologous Stem Cell Transplantation. This study enrolled 81 patients and patients received 28-day cycles of MONJUVI® 12 mg/kg IV once weekly during Cycles 1-3 with a loading dose on Cycle 1 Day 4, then every 2 weeks during Cycles 4-12, along with REVLIMID® 25 mg orally daily on Days 1-21 of Cycles 1-12. After Cycle 12, progression-free patients received MONJUVI® every 2 weeks until disease progression. Eighty patients (N=80) received at least one dose of both MONJUVI® and REVLIMID®. The Primary endpoint was Objective Response Rate (ORR). Secondary endpoints included Duration of Response (DoR), Progression-Free Survival (PFS) and Overall Survival (OS).
In this long-term analysis after a minimum of two years follow-up, outcomes from the L-MIND study were consistent with the primary analysis. Assessment by an Independent Review Committee at data cut-off showed an ORR of 58.8% and a Complete Response (CR) rate of 41.3%. Median Duration of Response was 34.6 months. The median OS was 31.6 months and median PFS was 16.2 months. The safety profile was consistent with that observed in previously reported studies of MONJUVI® in combination with REVLIMID®. The most common Grade 3 or worse Adverse Events were cytopenias and febrile neutropenia.
To determine the the contribution of MONJUVI® in the combination with REVLIMID® and to prove its synergistic effect, an observational retrospective study was conducted (Re-MIND) to compare real-world response data of patients with Relapsed or Refractory DLBCL who received REVLIMID® monotherapy with the efficacy outcomes of the MONJUVI®- REVLIMID® combination, as investigated in the L-MIND trial. In this study, efficacy data was collected from 490 R/R DLBCL patients in the US and EU. Qualification criteria for matching patients of both studies were pre-specified. As a result, 76 eligible Re-MIND patients were identified and matched 1:1 to 76 of 80 L-MIND patients based on important baseline characteristics. Objective response rates (ORR) were validated based on this subset of 76 patients in Re-MIND and L-MIND, respectively. The Primary endpoint of Re-MIND was met and shows a statistically significant superior best ORR of the MONJUVI®/ REVLIMID® combination compared to REVLIMID® monotherapy. Further, there was a significant difference in OS as well as CR rates, favoring the L-MIND cohort over the observational cohort.
It was concluded that MONJUVI® in combination with REVLIMID® resulted high Complete Response rates, as well Durable Responses and improved survival, in a significant proportion of patients with relapsed or refractory Diffuse Large B-Cell Lymphoma, ineligible for Autologous Stem Cell Transplantation, and might represent a new therapeutic option in this clinical setting.
Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Salles G, Duell J, Barca EG, et al. Lancet Oncol. 2020 Jul;21:978-988.
TECARTUS®
The FDA on July 24, 2020 granted accelerated approval to TECARTUS® (brexucabtagene autoleucel), a CD19-directed genetically modified Autologous T cell immunotherapy, for the treatment of adult patients with relapsed or refractory Mantle Cell Lymphoma (MCL). TECARTUS® is a product of Kite Pharma, a subsidiary of Gilead Sciences.
XPOVIO® (Selinexor)
The FDA on June 22,2020 granted accelerated approval to XPOVIO® for adult patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL), Not Otherwise Specified, including DLBCL arising from Follicular Lymphoma, after at least 2 lines of systemic therapy. XPOVIO® is a product of Karyopharm Therapeutics.
TAZVERIK® (Tazemetostat)
The FDA on June 18, 2020 granted accelerated approval to TAZVERIK®, an EZH2 inhibitor, for adult patients with Relapsed or Refractory (R/R) Follicular Lymphoma (FL) whose tumors are positive for an EZH2 mutation, as detected by an FDA-approved test, and who have received at least 2 prior systemic therapies, and for adult patients with R/R FL who have no satisfactory alternative treatment options. TAZVERIK® is a product of Epizyme, Inc.
IMBRUVICA® (Ibrutinib)
The FDA on April 21, 2020 expanded the indication of IMBRUVICA® to include its combination with RITUXAN® (Rituximab) for the initial treatment of adult patients with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). IMBRUVICA® is a product of Pharmacyclics LLC.
BRUKINSA® (Zanubrutinib)
The FDA on November 14, 2019 granted accelerated approval to BRUKINSA® for adult patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. BRUKINSA® is a product of BeiGene, Ltd.
FDA Approves Antibody-Drug Conjugate POLIVY® for Diffuse Large B-Cell Lymphoma
SUMMARY: The FDA on June 10, 2019, granted accelerated approval to POLIVY® (Polatuzumab vedotin-piiq), a CD79b-directed Antibody-Drug Conjugate, indicated in combination with Bendamustine and a Rituximab product, for adult patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL), Not Otherwise Specified, after at least two prior therapies.
The American Cancer Society estimates that in 2019, about 74,200 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 19,970 individuals will die of this disease. Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3-4% each year. More than half of patients are 65 or older at the time of diagnosis and the incidence is likely to increase with the aging of the American population. The etiology of Diffuse Large B-Cell Lymphoma is unknown. Contributing risk factors include immunosuppression (AIDS, transplantation setting, autoimmune diseases), UltraViolet radiation, pesticides, hair dyes, and diet. DLBCL is a neoplasm of large B cells and the most common chromosome abnormality involves alterations of the BCL-6 gene at the 3q27 locus, which is critical for germinal center formation. Two major molecular subtypes of DLBCL arising from different genetic mechanisms have been identified, using gene expression profiling: Germinal Center B-cell-like (GCB) and Activated B-Cell-like (ABC). Patients in the GCB subgroup have a higher five year survival rate, independent of clinical IPI (International Prognostic Index) risk score, whereas patients in the ABC subgroup have a significantly worse outcome. Regardless, R-CHOP regimen (RITUXAN®-Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone), given every 21 days, for 6 cycles, delivered with curative intent, is the current standard of care for patients of all ages, with newly diagnosed DLBCL, regardless of molecular subtype. Approximately 30-40% of patients experience disease progression or relapse, during the first 2 years and attempts to improve on R-CHOP regimen have not been successful. Maintenance treatment strategy following R-CHOP, to better control the disease, delay disease progression and improve long term survival, have included Autologous Stem Cell Transplantation, maintenance treatment with agents such as oral protein kinase inhibitor Enzastaurin and Everolimus. Outcomes for transplant-ineligible patients with Relapsed/Refractory DLBCL patients remain poor.
CD79b is a B-cell specific surface protein, which is a component of the B-cell receptor. POLIVY® is a CD79b-directed Antibody-Drug Conjugate (ADC) with activity against dividing B cells. It consists of three components: 1) the humanized ImmunoGlobulin G1 (IgG1) monoclonal antibody specific for human CD79b; 2) the small molecule anti-mitotic agent MMAE (monomethyl auristatin E) and 3) a protease-cleavable linker that covalently attaches MMAE to the Polatuzumab antibody. Upon binding to CD79b, POLIVY® is internalized, and the linker is cleaved by lysosomal proteases thus enabling intracellular delivery of MMAE. MMAE then binds to microtubules and kills dividing cells by inhibiting cell division and inducing apoptosis.
The present FDA approval was based on an open-label, randomized, multicenter, Phase II clinical trial (Study GO29365) which included a cohort of 80 transplant-ineligible patients with Relapsed or Refractory DLBCL. Patients who had received at least one prior regimen were randomized (1:1) to receive either POLIVY® in combination with Bendamustine and RITUXAN® (Rituximab) or GAZYVA® (Obinutuzumab) – (P+BR) or BR alone, every 21 days for up to 6 cycles. RITUXAN® or GAZYVA® were administered on day 1 of each cycle at 375 mg/m2 intravenously IV or 1000 mg IV respectively, POLIVY® 1.8 mg/kg IV, was given on day 2 of cycle 1 and on day 1 of subsequent cycles and Bendamustine 90 mg/m2 IV was administered on days 2 and 3 of cycle 1 and on days 1 and 2 of subsequent cycles. The median age was 68 years.
The Primary aim of this study was to assess the efficacy of P+BR versus BR at end of treatment, by an Independent Review Committee (IRC). Responses were assessed using the modified Lugano Classification, and Complete Response (CR) required Positron Emission Tomography (PET) negativity and negative bone marrow biopsy, if positive or unknown at the time of screening. Other end points included Duration of Response (DoR), Progression Free Survival (PFS) and Overall Survival (OS). Efficacy was also evaluated based on Cell of Origin – Activated B-cell-like (ABC), Germinal B-cell-like (GCB), as well as MYC/BCL2 double expression. The median follow up for this cohort of patients was 22.3 months.
The combination of POLIVY® plus BR (P+BR) showed significantly higher PET-CR rates vs BR alone (40% vs 18%; P=0.026). The Objective Response Rate (ORR) was 45% vs 18% with a significantly longer DoR of 10.3 months vs 4.1 months (HR=0.44; P=0.032), favoring P+BR regimen. Among those who achieved Partial or Complete Response to P+BR, 64% had response durations of at least six months and 48% had response durations of at least 12 months. The PFS was 7.6 months vs 2 months (HR=0.34; P<0.0001) and OS was 12.4 months vs 4.7 months (HR=0.42; P=0.0023), all favoring P+BR over BR. For ABC patients the median PFS with P+BR was 10.5 months vs 2.5 months for BR and the median OS was 13.9 vs 4.3 months, respectively. For GCB patients, median PFS with P+BR was 4.7 vs 1.5 months for BR and the median OS was 9.3 vs 3.2 months, respectively. Among those with MYC/BCL2 Double Expression, the median PFS was 7.0 months vs 0.7 months with P+BR, and median OS was 12.9 vs 3.8 months compared to BR. For non-Double Expression group of patients, the median PFS was 6.3 months vs 2.5 months and median OS was 10.5 vs 3.8 months with P+BR vs BR, respectively.
It was concluded that a combination of POLIVY® given along with Bendamustine and RITUXAN® or GAZYVA® provides a promising treatment option for Relapsed/Refractory DLBCL patients who are transplant ineligible, with durables responses in some patients of over 20 months and median OS surpassing 12 months. Polatuzumab vedotin (Pola) plus bendamustine (B) with rituximab (R) or obinutuzumab (G) in relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL): updated results of a phase (Ph) Ib/II study. Sehn LH, Herrera AF, Matasar MJ, et al. Blood. 2018;132:1683.
POLIVY® (Polatuzumab vedotin-piiq)
The FDA on June 10, 2019 granted accelerated approval to POLIVY®, a CD79b-directed Antibody-Drug Conjugate indicated in combination with Bendamustine and a Rituximab product for adult patients with relapsed or refractory Diffuse Large B-Cell Lymphoma (DLBCL), Not Otherwise Specified, after at least two prior therapies. POLIVY® is a product of Genentech, Inc.
REVLIMID® plus RITUXAN® (R2) Significantly Improves Progression Free Survival in Relapsed or Refractory Indolent Lymphoma
SUMMARY: The American Cancer Society estimates that in 2019, about 74,200 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 19,970 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT), Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 20% of all NHLs are Follicular Lymphomas (FL).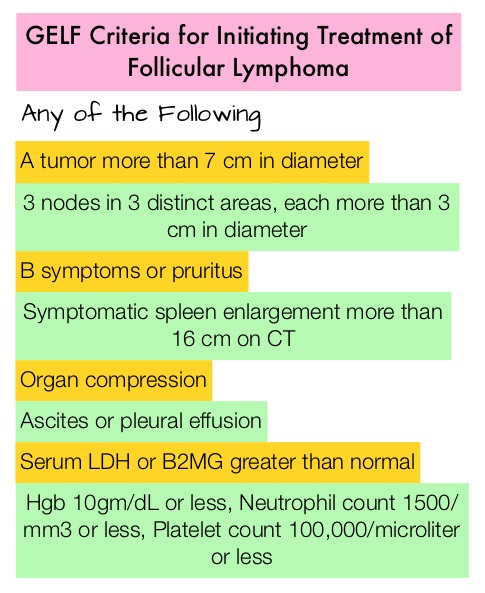
The Ann Arbor classification divides FL into four stages. Patients with Stages I and II have localized disease and those with Stages III and IV have advanced disease. The World Health Organization (WHO) further classified FL based on histology into low grade (grades 1 and 2) and high grade (grade 3a) FLs. Grade 3b FL which demonstrates diffuse areas of involvement is designated as Diffuse Large B-cell Lymphoma (DLBCL) and is treated as such. Advanced stage indolent NHL is not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life, have been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach. Patients with advanced stage symptomatic Follicular Lymphoma are often treated with induction chemoimmunotherapy followed by maintenance RITUXAN® (Rituximab). This can result in a median PFS of 6-8 yrs and a median OS of 12-15 yrs. However, approximately 30% of the patients will relapse in 3 years and treatment options are limited for patients with relapses, after multiple treatments.
REVLIMID® (Lenalidomide) is an oral immunomodulatory agent (IMiD) with activity in lymphoid malignancies, primarily through immune modulation, by reactivating and heightening immune system responses to malignant cells. REVLIMID® synergizes with anti-CD20 antibodies such as RITUXAN® and GAZYVA® (Obinutuzumab) and enhances the function of T and NK cells, increases Antibody-Dependent Cellular Cytotoxicity (ADCC), and repair defective synapse formation in B-cell lymphoma cells, thereby restoring the immune system’s ability to kill tumor cells, without permanently damaging the healthy microenvironment, or causing long-term immune suppression. Chemo-free combination immunotherapy with REVLIMID® and RITUXAN® or the R2 regimen, has shown promising activity in phase II studies. RELEVANCE phase III trial compared REVLIMID® plus RITUXAN®, followed by RITUXAN® maintenance, with the standard of care treatment of RITUXAN® plus chemotherapy, followed by RITUXAN® maintenance, in patients with previously untreated Follicular Lymphoma and concluded that R2 regimen showed similar efficacy, with a more favorable safety profile, making it a potential chemo-free first line option, for patients with Follicular Lymphoma. Single agent RITUXAN® is commonly used in the second-line treatment of Follicular Lymphoma (25-47% of patients), according to studies in the United States and Europe.
AUGMENT trial is a prospective, Phase III, multicenter, randomized study in which 358 patients with relapsed or refractory grades 1-3a Follicular Lymphoma (82%) or Marginal Zone Lymphoma (18%) were randomly assigned to receive either REVLIMID® plus RITUXAN® (N=178) or placebo plus RITUXAN® (N=180). Eligible patients had at least one prior chemotherapy, immunotherapy, or chemoimmunotherapy and two or more previous doses of RITUXAN®, and had relapsed, refractory, or progressive disease, but not RITUXAN®-refractory. Patients with greater than grade 1 neuropathy were excluded. The median age was 63 years, over 50% of the patients had high tumor burden based on GELF criteria, and over 40% of patients had at least 2 prior systemic regimens. REVLIMID® plus RITUXAN® regimen consisted of REVLIMID® 20 mg orally daily (10 mg for Creatinine Clearance 30-59 mL/min) on days 1 to 21 plus RITUXAN® 375 mg/m2 IV on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 2 to 5 every 28 days for 12 cycles. Placebo plus RITUXAN® was administered similarly. The Primary end point was PFS assessed by the Independent Review Committee (IRC) and Secondary end points included Overall Response Rate (ORR), Complete Response (CR), Duration of Response, Overall Survival (OS), and time to next anti-lymphoma treatment. The median follow-up was 28.3 months.
The median PFS was 39.4 months in the REVLIMID®/ RITUXAN® group versus 14.1 months in the placebo plus RITUXAN® group (HR=0.46, P<0.001). This meant a reduced risk of disease progression by 54% and increased median PFS by more than 2 years compared with RITUXAN® monotherapy. This benefit was consistent across all prespecified subgroups, except for the Marginal Zone Lymphoma subgroup and it may be difficult to draw strong conclusions in this small subgroup because these patients made up only 18% of the overall patient population. Response rates as assessed by IRC were 78% in the REVLIMID®/ RITUXAN® group versus 53% in the placebo plus RITUXAN® group (P<0.001), with Complete Response occurring in 34% versus 18% respectively (P=0.001). The median Duration of Response was 36.6 versus 21.7 months (P =0.0015), median Event-Free Survival was 27.6 versus 13.9 months (HR = 0.51, P<0.001) and the median time to next anti-lymphoma treatment was Not Reached versus 32.2 months (P=0.007).
It was concluded that the R2 regimen (REVLIMID® plus RITUXAN®) significantly prolonged Progression Free Survival in patients with relapsed or refractory indolent lymphoma and represents an important new treatment option for this patient group. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. Leonard JP, Trneny M, Izutsu K, et al. J Clin Oncol 2019;37:1188-1199.
