SUMMARY: Aspirin (AcetylSalicylic Acid) has been studied as a chemopreventive agent for several decades and the temporal relationship between systemic inflammation and cancer has been a topic of ongoing investigation. The US Preventive Services Task Force (USPSTF) found adequate evidence that Aspirin use reduces the incidence of ColoRectal Cancer (CRC) in adults after 5-10 years of use, and recommends initiating low-dose Aspirin use for the primary prevention of CardioVascular Disease (CVD) and ColoRectal Cancer (CRC) in adults aged 50-69 years, who have a 10% or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose Aspirin daily for at least 10 years.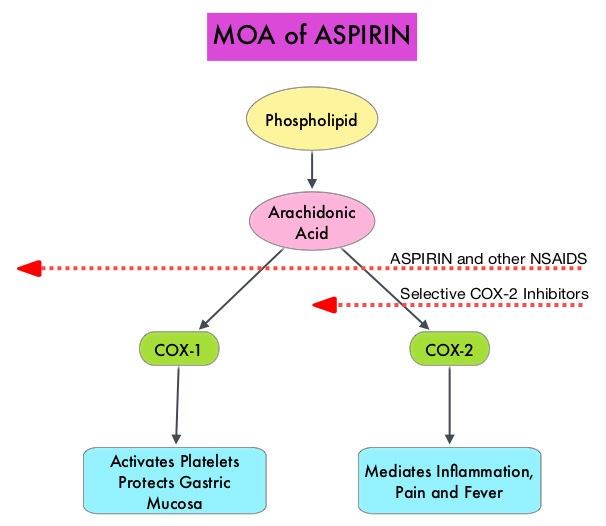
The molecular mechanisms underlying Aspirin’s chemoprevention effects as well as the dose, duration, and timing of Aspirin chemoprevention have remained unclear. More recent data suggests that platelets may play a role in tumorigenesis as well, through the release of angiogenic and growth factors due to overexpression of COX-2. Daily low dose Aspirin inhibits COX-1 and COX-2. It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties.
Two recently published studies examining different doses of Aspirin in different cancers, highlight the beneficial role of Aspirin in reducing cancer risk.
The first report is a large prospective study which attempted to reproduce findings from case-control studies that reported lower Ovarian cancer risk among low-dose Aspirin users. This prospective study evaluated whether regular Aspirin or nonaspirin NonSteroidal Anti-Inflammatory Drug (NSAID) use and patterns of use was associated with lower risk of Ovarian cancer. This cohort study analyzed NSAID use and Ovarian cancer diagnosis data on 205,498 women from 2 prospective cohorts; 93 664 women in the Nurses’ Health Study (NHS), who were 93% non-Hispanic white, with a mean age at baseline of 46 years, followed up from 1980 to 2014, and 111834 women in the Nurses’ Health Study II (NHSII), who were 92% non-Hispanic white, with a mean age at baseline of 34.2 years, followed up from 1989 to 2015. For each analgesic type (Aspirin, low-dose Aspirin, nonaspirin NSAIDs, and Acetaminophen), timing, duration, frequency, and number of tablets used were evaluated, and information was updated every 2-4 years.
It was noted that among both cohorts, there were 1054 women who developed epithelial Ovarian cancer. Recent use of low-dose Aspirin (100 mg or less) was associated with a lower risk of Ovarian cancer (HR=0.77), whereas there was no such association noted with standard-dose of 325 mg Aspirin (HR=1.17). The associations between Aspirin use and risk of Ovarian cancer did not differ among premenopausal versus postmenopausal women. This study also suggested that use of non-aspirin NSAIDs, such as Ibuprofen and Naproxen, when taken in quantities of at least 10 tablets per week for multiple years was positively associated with an increased risk of Ovarian cancer. There was however no clear associations for the use of Acetaminophen.
The authors concluded that consistent with case-control studies, this prospective analysis showed a reduced risk of Ovarian cancer among regular users of low-dose Aspirin and an increased risk of Ovarian cancer with the use of nonaspirin NSAIDs. These findings suggest that low-dose Aspirin recommended for cardiovascular prophylaxis and ColoRectal Cancer risk reduction can also reduce the risk of Ovarian cancer.
The second report is another large prospective study which analyzed the data on the risk of HepatoCellular Carcinoma (HCC) within 2 populations of a total of 133 371 health care professionals who self reported use of Aspirin. In this pooled analysis, 87 507 were women, with a mean age was 62 years, and 45 864 were men, with a mean age of 64 years. Women reported data biennially since 1980 and men since 1986, on frequency, dosage, and duration of Aspirin use, and data were accessed from November 2017 through March 2018. Individuals with a cancer diagnosis at baseline (except nonmelanoma skin cancer) were excluded.
The researchers noted that regular Aspirin use of 2 or more standard dose 325 mg tablets per week was associated with a 49% reduction in risk of HCC (adjusted HR=0.51) compared to non regular use. This benefit was dose-dependent with the greatest benefit among those taking more than 5 tablets per week (P for trend =0 .006). Further, significantly lower risk for HCC was observed with increasing duration of Aspirin intake (P for trend =0.03), with this decreasing risk noted with the use of 1.5 or more standard-dose Aspirin tablets per week for 5 or more years (adjusted HR=0.41). The use of nonaspirin NSAIDs however was not significantly associated with HCC risk.
The authors from this study concluded that regular and long-term use of standard dose (325 mg) Aspirin, taken at least 2 or more times per week is associated with a dose-dependent reduction in HCC risk, which is apparent after 5 or more years of use.
Taken together, these 2 studies provide the evidence supporting the ability of regular use of Aspirin to prevent Ovarian cancer and HepatoCellular Cancer (HCC). Aspirin is rapidly emerging as a valuable chemoprevention agent for various malignancies.
Association of Analgesic Use With Risk of Ovarian Cancer in the Nurses’ Health Studies. Barnard ME, Poole EM, Curhan GC, et al. JAMA Oncol. 2018;4:1675-1682.
Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. Simon TG, Ma Y, Ludvigsson JF, et al. JAMA Oncol. 2018;4:1683-1690