SUMMARY:Carcinoma of Unknown Primary Site (CUPS) is a heterogeneous clinical pathologic syndrome for which the anatomical site of origin of the primary tumor is clinically undetectable. CUPS accounts for approximately 2% of all advanced malignances annually. The American Cancer Society estimates that about 31,430 cases of Cancer of Unknown Primary site will be diagnosed in 2014 in the United States. The pathobiology of tumors from unknown primary sites is similar to those with detectable primary tumors and therefore may respond to therapies similar to those with easily detectable primary tumors. Historically, the treatment approach for patients with CUPS included broad spectrum empiric chemotherapy. Histological evaluation of the biopsy tissue alone has been the standard practice for decades. With the availability of gene expression profiling assays and advances in ImmunoHistoChemistry staining as well as imaging technology, predicting the tissue of origin of the primary tumor and tailoring therapy accordingly, has improved overall survival in this patient population. Evaluation of a patient with CUPS starts with gathering and incorporating medical information which includes the patient’s gender, medical history, clinical findings and sites of metastases. A CT scan of the chest, abdomen and pelvis with IV and oral contrast is recommended, although PET (Positron Emission Tomography) or an MRI can be performed in those with renal insufficiency or iodine allergy. PET scan is recommended for those with cervical lymphadenopathy with squamous histology, to help determine the extent of the disease and treatment planning for radiation. PET imaging is also helpful for patients with solitary metastases before locoregional therapies are planned, as well as assessing response in patients with predominantly bone only disease. In women presenting with isolated axillary lymphadenopathy, adenocarcinoma histology, negative mammograms and ultrasound, MRI of the breasts is indicated. With the exception of those patients with CUPS who present with cervical lymphadenopathy, diagnostic procedures such as bronchoscopy, EGD and colonoscopy are not recommended in asymptomatic patients. Tumor markers in general do not have diagnostic value in patients with CUPS although they could be utilized to monitor response to treatment. However, PSA when elevated in a male with adenocarcinoma and osteoblastic metastases, is suggestive of a prostate primary. Similarly an elevated Beta HCG and AFP in a patient with undifferentiated or poorly differentiated carcinoma, is suggestive of an extragonadal germ cell tumor and an elevated AFP is also helpful in making a diagnosis of Hepatoma. Approximately 60% of the patients with CUPS have well or moderately differentiated adenocarcinoma on light microscopy, 30% have poorly differentiated carcinoma or adenocarcinoma, 5% have poorly differentiated or undifferentiated malignancy and 5% have squamous cell carcinoma. Following histological evaluation on light microscopy, the biopsy specimen is further tested using ImmunoHistoChemical stains, using peroxidase labeled antibodies against tumor specific antigens, taking advantage of the similarities in the tumor profiles of primary and metastatic malignancies. After delineating a tumor as carcinoma, lymphoma, sarcoma or melanoma, additional IHC testing can help identify tumors such as a lung primary (postive Thyroid Transcription Factor 1-TTF1and positive CytoKeratin 7- CK7), lower gastrointestinal cancers (positive CK20, positive CDX2 and negative CK7) or a breast primary (positive CK7 and positive Mammaglobin). Tissue-of-Origin molecular profiling is based on the principle that in patients with CUPS, molecular signatures of metastatic tumors are similar to their primary tumor. Tissue-of-Origin molecular profiling is performed using tools such as DNA microarray, quantitative real time polymerase chain reaction assay (rt-PCR) or assays based on messenger RNA (mRNA) or microRNA. These tests are cost-effective and 70% – 90% accurate. This study can be performed on formalin-fixed samples as well as samples from fine needle aspiration. Even though platinum based chemotherapy has been the default regimen for patients with CUPS, histological evaluation of biopsy tissue by light microscopy, IHC testing and molecular profiling assay may complement each other and help guide the Health Care Provider to select site specific therapy. The survival outcomes of CUPS patients with a Tissue-of-Origin molecularly diagnosed profile are comparable to those with similar type advanced cancer with a known primary. The authors concluded that with additional molecular insights into tumor biology and availability of newer therapeutic agents, patients with CUPS and known primary tumors may eventually be treated alike. Varadhachary, GR and Raber, MN. N Engl J Med 2014; 371:757-765
Evaluation of a patient with CUPS starts with gathering and incorporating medical information which includes the patient’s gender, medical history, clinical findings and sites of metastases. A CT scan of the chest, abdomen and pelvis with IV and oral contrast is recommended, although PET (Positron Emission Tomography) or an MRI can be performed in those with renal insufficiency or iodine allergy. PET scan is recommended for those with cervical lymphadenopathy with squamous histology, to help determine the extent of the disease and treatment planning for radiation. PET imaging is also helpful for patients with solitary metastases before locoregional therapies are planned, as well as assessing response in patients with predominantly bone only disease. In women presenting with isolated axillary lymphadenopathy, adenocarcinoma histology, negative mammograms and ultrasound, MRI of the breasts is indicated. With the exception of those patients with CUPS who present with cervical lymphadenopathy, diagnostic procedures such as bronchoscopy, EGD and colonoscopy are not recommended in asymptomatic patients. Tumor markers in general do not have diagnostic value in patients with CUPS although they could be utilized to monitor response to treatment. However, PSA when elevated in a male with adenocarcinoma and osteoblastic metastases, is suggestive of a prostate primary. Similarly an elevated Beta HCG and AFP in a patient with undifferentiated or poorly differentiated carcinoma, is suggestive of an extragonadal germ cell tumor and an elevated AFP is also helpful in making a diagnosis of Hepatoma. Approximately 60% of the patients with CUPS have well or moderately differentiated adenocarcinoma on light microscopy, 30% have poorly differentiated carcinoma or adenocarcinoma, 5% have poorly differentiated or undifferentiated malignancy and 5% have squamous cell carcinoma. Following histological evaluation on light microscopy, the biopsy specimen is further tested using ImmunoHistoChemical stains, using peroxidase labeled antibodies against tumor specific antigens, taking advantage of the similarities in the tumor profiles of primary and metastatic malignancies. After delineating a tumor as carcinoma, lymphoma, sarcoma or melanoma, additional IHC testing can help identify tumors such as a lung primary (postive Thyroid Transcription Factor 1-TTF1and positive CytoKeratin 7- CK7), lower gastrointestinal cancers (positive CK20, positive CDX2 and negative CK7) or a breast primary (positive CK7 and positive Mammaglobin). Tissue-of-Origin molecular profiling is based on the principle that in patients with CUPS, molecular signatures of metastatic tumors are similar to their primary tumor. Tissue-of-Origin molecular profiling is performed using tools such as DNA microarray, quantitative real time polymerase chain reaction assay (rt-PCR) or assays based on messenger RNA (mRNA) or microRNA. These tests are cost-effective and 70% – 90% accurate. This study can be performed on formalin-fixed samples as well as samples from fine needle aspiration. Even though platinum based chemotherapy has been the default regimen for patients with CUPS, histological evaluation of biopsy tissue by light microscopy, IHC testing and molecular profiling assay may complement each other and help guide the Health Care Provider to select site specific therapy. The survival outcomes of CUPS patients with a Tissue-of-Origin molecularly diagnosed profile are comparable to those with similar type advanced cancer with a known primary. The authors concluded that with additional molecular insights into tumor biology and availability of newer therapeutic agents, patients with CUPS and known primary tumors may eventually be treated alike. Varadhachary, GR and Raber, MN. N Engl J Med 2014; 371:757-765
Tag: General Medical Oncology & Hematology
Phase 3 study of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting during repeated moderately emetogenic chemotherapy (MEC) cycles
SUMMARY: Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. The development of effective antiemetic agents has facilitated the administration of majority of the chemotherapy agents in an outpatient setting avoiding hospitalization. Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist. Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Electronic Cigarettes A Position Statement of the Forum of International Respiratory Societies
SUMMARY: According to the American Cancer Society, tobacco use is responsible for nearly 1 in 5 deaths in the United States and accounts for at least 30% of all cancer deaths. Smokeless tobacco products are a major source of cancer causing nitrosamines and increase the risk of developing cancer of the oropharynx, esophagus, and pancreas. Cigarette smoke contains more than 7,000 chemicals, many of which are toxic and some linked to cancer. E-cigarettes were first developed in China and were introduced to the U.S. market in 2007. When a smoker inhales through the mouth piece of an E-cigarette, the air flow triggers a sensor that switches on a small lithium battery powered heater, which in turn vaporizes liquid nicotine along with PolyEthylene Glycol (PEG) present in a small cartridge. The PEG vapor looks like smoke. The potent liquid form of nicotine extracted from tobacco is tinctured with fragrant flavors such as chocolate, cherry and bubble gum, coloring substances, as well other chemicals and these e-liquids are powerful neurotoxins. With the rapid growth of the E-cigarette industry and the evidence of potential dangers and risk to public health, particularly children, experts from the world's leading lung organizations were compelled to release a position statement on electronic cigarettes, specifically focusing on their potential adverse effects on human health and calling on government organizations to ban or restrict the use of E-cigarettes, until their impact on health is better understood. With epidemiological data demonstrating that nicotine use is a gateway to the use of cocaine and marijuana and subsequent lifelong addiction, the Forum of International Respiratory Societies (FIRS), an organization composed of the world's leading international respiratory societies including American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP), made the following recommendations. The position statement of the Forum of International Respiratory Societies (FIRS) on electronic nicotine delivery devices includes the following:
When a smoker inhales through the mouth piece of an E-cigarette, the air flow triggers a sensor that switches on a small lithium battery powered heater, which in turn vaporizes liquid nicotine along with PolyEthylene Glycol (PEG) present in a small cartridge. The PEG vapor looks like smoke. The potent liquid form of nicotine extracted from tobacco is tinctured with fragrant flavors such as chocolate, cherry and bubble gum, coloring substances, as well other chemicals and these e-liquids are powerful neurotoxins. With the rapid growth of the E-cigarette industry and the evidence of potential dangers and risk to public health, particularly children, experts from the world's leading lung organizations were compelled to release a position statement on electronic cigarettes, specifically focusing on their potential adverse effects on human health and calling on government organizations to ban or restrict the use of E-cigarettes, until their impact on health is better understood. With epidemiological data demonstrating that nicotine use is a gateway to the use of cocaine and marijuana and subsequent lifelong addiction, the Forum of International Respiratory Societies (FIRS), an organization composed of the world's leading international respiratory societies including American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP), made the following recommendations. The position statement of the Forum of International Respiratory Societies (FIRS) on electronic nicotine delivery devices includes the following:
• The safety of electronic cigarettes has not been adequately demonstrated.
• The addictive power of nicotine and its untoward effects should not be underestimated.
• The potential benefits of electronic nicotine delivery devices, including harm reduction and as an aid to smoking cessation, have not been well studied.
• Potential benefits to an individual smoker should be weighed against harm to the population of increased social acceptability of smoking and use of nicotine.
• Health and safety claims regarding electronic nicotine delivery devices should be subject to evidentiary review.
• Adverse health effects for third parties exposed to the emissions of electronic cigarettes cannot be excluded.
• Electronic nicotine delivery devices should be restricted or banned, at least until more information about their safety is available.
• If electronic nicotine delivery devices are permitted, they should be regulated as medicines and subject to the same evidentiary review of other medicines.
• If electronic nicotine delivery devices are not regulated as medicines, they should be regulated as tobacco products.
• Research, supported by sources other than the tobacco or electronic cigarette industry, should be carried out to determine the impact of electronic nicotine delivery devices on health in a wide variety of settings.
• The use and population effects of electronic nicotine delivery devices should be monitored.
• All information derived from this research should be conveyed to the public in a clear manner.
Schraufnagel DE, Blasi F, Drummond MB, et al. on behalf of the Forum of International Respiratory Societies. Am J Respir Crit Care Med. First published online 09 Jul 2014 as DOI: 10.1164/rccm.201407-1198PP
Calcium, Vitamin D, Dairy Products, and Mortality Among Colorectal Cancer Survivors The Cancer Prevention Study-II Nutrition Cohort
SUMMARY: The American Cancer Society estimates that approximately 137,000 new cases of colorectal cancer will be diagnosed in the United States in 2014 and over 50,000 are expected to die of the disease. Data from 60 epidemiological studies enrolling more than 26,000 ColoRectal Cancer (CRC) patients have shown that higher consumption of milk and dairy products reduces the risk of colon cancer and high Calcium intake reduces the risk of CRC. In vivo and in vitro studies have confirmed these findings.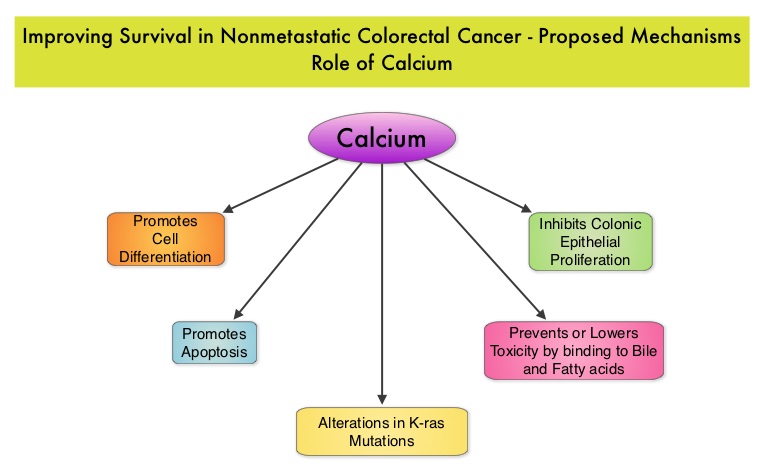 Milk, in addition to being a rich source of dietary Calcium and Vitamin D is a primary dietary source of conjugated linoleic acid which has been shown to inhibit colon cancer cell growth. Dairy products also provide other beneficial components such as butyric acid, lactoferrin and fermentation products. The impact of milk and dairy products on CRC survival however has remained unclear. The Cancer Prevention Study – II Nutrition Cohort is a prospective study of cancer incidence that began in 1992. Participants in this study (N=184,000) were provided a self administered questionnaire and baseline information about their dietary habits (including dietary Calcium and Vitamin D, as well as Calcium, Vitamin D and multivitamin supplements), physical activity, body size, cancer screening and early detection, etc. was collected and follow up questionnaires were sent every other year to update information and learn about new cancer diagnosis. Patients were followed up until June 2009 and by the end of this period, 3,832 individuals who had no history of disease at baseline had been diagnosed with invasive colon or rectal cancer. After excluding patients with distant metastatic disease, 2,284 patients were included in this analysis and among them, 1,111 patients reported post diagnosis diet. The primary outcome of this study was all cause mortality and the secondary outcome was mortality resulting from colorectal cancer. Using standard statistical models, the investigators noted that post CRC diagnosis total Calcium intake and milk intake, was inversely associated with all-cause mortality and significantly reduced CRC specific mortality.
Milk, in addition to being a rich source of dietary Calcium and Vitamin D is a primary dietary source of conjugated linoleic acid which has been shown to inhibit colon cancer cell growth. Dairy products also provide other beneficial components such as butyric acid, lactoferrin and fermentation products. The impact of milk and dairy products on CRC survival however has remained unclear. The Cancer Prevention Study – II Nutrition Cohort is a prospective study of cancer incidence that began in 1992. Participants in this study (N=184,000) were provided a self administered questionnaire and baseline information about their dietary habits (including dietary Calcium and Vitamin D, as well as Calcium, Vitamin D and multivitamin supplements), physical activity, body size, cancer screening and early detection, etc. was collected and follow up questionnaires were sent every other year to update information and learn about new cancer diagnosis. Patients were followed up until June 2009 and by the end of this period, 3,832 individuals who had no history of disease at baseline had been diagnosed with invasive colon or rectal cancer. After excluding patients with distant metastatic disease, 2,284 patients were included in this analysis and among them, 1,111 patients reported post diagnosis diet. The primary outcome of this study was all cause mortality and the secondary outcome was mortality resulting from colorectal cancer. Using standard statistical models, the investigators noted that post CRC diagnosis total Calcium intake and milk intake, was inversely associated with all-cause mortality and significantly reduced CRC specific mortality.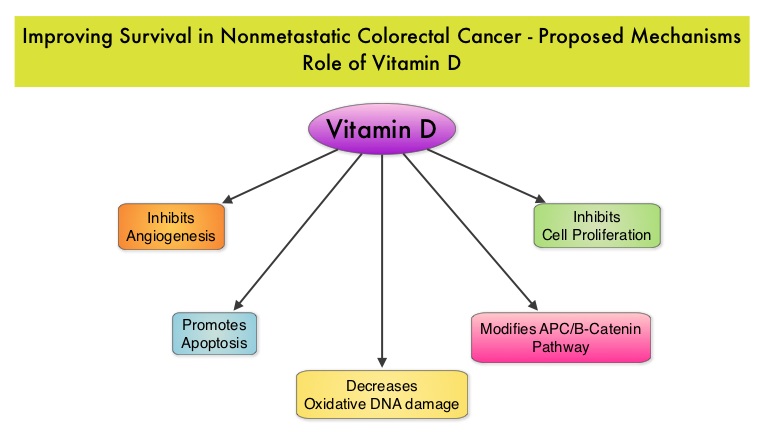 This benefit however, was not seen with Vitamin D intake. Also of interest, pre-diagnosis Calcium, Vitamin D, and dairy product intakes did not influence mortality outcomes. The authors concluded that higher post-diagnosis intakes of total Calcium and milk may be associated with lower risk of death among patients with non-metastatic ColoRectal Cancer. In a more recent publication, it has been reported that there is a strong association between plasma level of 25-hydroxyvitamin D (25-OHD) and CRC specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25-OHD (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). With 30-35% of the malignancies attributed to dietary habits, the onus is therefore on the treating physicians to provide nutrition counseling during and after cancer treatment and is not to be ignored. Yang B, McCullough ML, Gapstur SM, et al. J Clin Oncol 2014;32:2335-2343
This benefit however, was not seen with Vitamin D intake. Also of interest, pre-diagnosis Calcium, Vitamin D, and dairy product intakes did not influence mortality outcomes. The authors concluded that higher post-diagnosis intakes of total Calcium and milk may be associated with lower risk of death among patients with non-metastatic ColoRectal Cancer. In a more recent publication, it has been reported that there is a strong association between plasma level of 25-hydroxyvitamin D (25-OHD) and CRC specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25-OHD (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). With 30-35% of the malignancies attributed to dietary habits, the onus is therefore on the treating physicians to provide nutrition counseling during and after cancer treatment and is not to be ignored. Yang B, McCullough ML, Gapstur SM, et al. J Clin Oncol 2014;32:2335-2343
NCCN Guidelines for Survivorship Expanded to Address Two Common Conditions
SUMMARY: The National Comprehensive Cancer Network (NCCN) has expanded its Survivorship Guidelines to include cancer-associated cognitive impairment and Chemotherapy Induced Peripheral Neuropathy. The later is a component of the Adult Cancer Pain section. Dr. Urba discussed the management of Chemotherapy Induced Peripheral Neuropathy at the NCCN 19th annual conference. Approximately 20%-40% of the patients suffer from Chemotherapy Induced Peripheral Neuropathy, which can result in premature discontinuation of treatment. Further, this adverse event in a significant number of patients can persist indefinitely and can be disabling, thus impacting their activities of daily living. The following chemotherapeutic agents are associated with varying degrees of peripheral neuropathy – Platinum compounds (Cisplatin, Carboplatin and Oxaliplatin), Taxanes (Paclitaxel, Docetaxel), Immunomodulatory agents (Thalidomide, Lenalidomide), Other Microtubule inhibitors (Vincristine, Ixabepilone) and Proteosome Inhibitors (Bortezomib). It may be necessary to screen and rescreen patients for neuropathic pain, as patients may not be forthcoming with this complaint. Management of Neuropathic pain may include systemic treatment with adjuvant analgesics, topical therapies and psychosocial support. The management of Chemotherapy Induced Peripheral Neuropathy has mostly been extrapolated from validated studies on diabetic neuropathy. The first line treatment for Chemotherapy Induced Neuropathic Pain includes antidepressants and anticonvulsants, which if not effective on their own, can be combined with opioids. TriCyclic Antidepressants (TCA’s) such as Amitriptyline and Nortriptyline (PAMELOR®) can be considered as first line choice for appropriate patients, although its mechanism of action is uncertain and 20% of the patients discontinue therapy because of adverse effects.
The following chemotherapeutic agents are associated with varying degrees of peripheral neuropathy – Platinum compounds (Cisplatin, Carboplatin and Oxaliplatin), Taxanes (Paclitaxel, Docetaxel), Immunomodulatory agents (Thalidomide, Lenalidomide), Other Microtubule inhibitors (Vincristine, Ixabepilone) and Proteosome Inhibitors (Bortezomib). It may be necessary to screen and rescreen patients for neuropathic pain, as patients may not be forthcoming with this complaint. Management of Neuropathic pain may include systemic treatment with adjuvant analgesics, topical therapies and psychosocial support. The management of Chemotherapy Induced Peripheral Neuropathy has mostly been extrapolated from validated studies on diabetic neuropathy. The first line treatment for Chemotherapy Induced Neuropathic Pain includes antidepressants and anticonvulsants, which if not effective on their own, can be combined with opioids. TriCyclic Antidepressants (TCA’s) such as Amitriptyline and Nortriptyline (PAMELOR®) can be considered as first line choice for appropriate patients, although its mechanism of action is uncertain and 20% of the patients discontinue therapy because of adverse effects.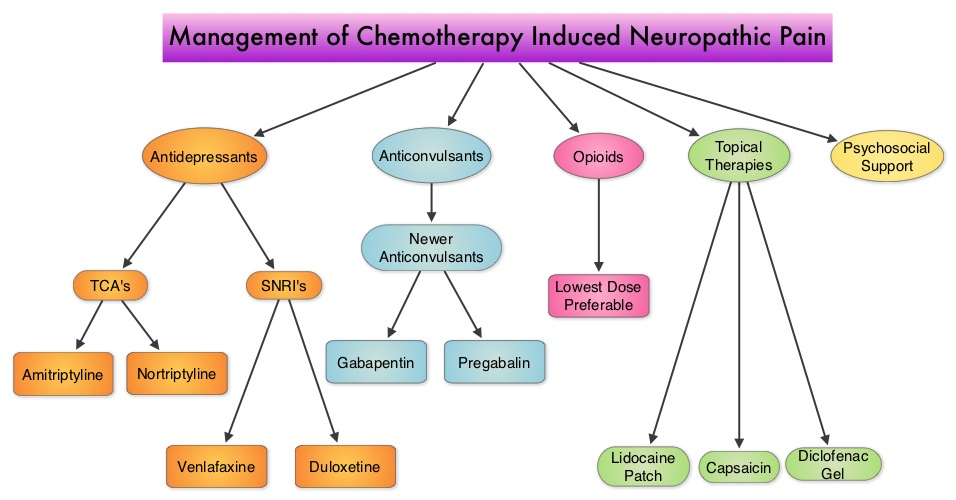 Patients may be able to better tolerate these agents if started at a lower dose and dose titrated slowly every three to five days. Peripheral neuropathic pain has been attributed to an unbalanced release of norepinephrine and serotonin from the neurons. Serotonin-Norepinephrine Reuptake Inhibitors (SNRI’s), including Venlafaxine (EFFEXOR®) and Duloxetine (CYMBALTA®), are better tolerated and have fewer drug interactions than TCA’s. EFFEXOR® in one study significantly relieved Oxaliplatin induced neuropathic pain in more than two thirds of the patients when compared to placebo and a third of the patients had complete pain relief. There is however limited evidence showing a beneficial role of Selective Serotonin Reuptake Inhibitors (SSRI’s) such as Citalopram (CELEXA®) and Paroxetine (PAXIL®) for neuropathic pain. As a note of caution, SNRI’s such as EFFEXOR® and CYMBALTA®, can interact with Tamoxifen, prescribed to patients with breast cancer, preventing Tamoxifen from converting to its active form. The dose of antidepressants needed to alleviate neuropathic pain is not dependent on antidepressant activity and may be lower than that recommended for treatment of depression. The newer anticonvulsants such as Gabapentin (NEURONTIN®), Pregabalin (LYRICA®) are preferable first line agents for the treatment of neuropathic pain rather than traditional, older agents such as Carbamazepine (TEGRETOL®), Phenytoin (DILANTIN®) and Valproate (DEPAKOTE®), as the newer agents are associated with fewer drug interactions. The newer agents bind to the alpha2-delta subunit of the calcium-sensitive channels, modulating neurotransmitter release. Of the newer agents, NEURONTIN® (Gabapentin) is not protein bound and is excreted unchanged in the urine and therefore has fewer drug interactions. If opioids are a consideration for neuropathic pain relief, the lowest dose is recommended. Topical therapies for neuropathic pain have the advantage of controlling pain without systemic side effects. It therefore can be combined with systemic treatment. Lidocaine 5% patches (LIDODERM®) block neuronal sodium channels whereas Capsaicin cream (ZOSTRIX®) stimulates the C fibers to release and subsequently deplete substance P, there by blocking pain signaling to the brain. Diclofenac gel 1% when applied once a day, concentrates in the dermis and has less gastrointestinal side effects and may be beneficial for neuropathic pain. A combination of Ketamine 1% and Amitriptyline 2% cream applied topically has also been promising in a small study. Patients experiencing refractory pain may benefit with the use of Transcutaneous Electrical Nerve Stimulation (TENS), although referral to the pain clinic may be appropriate. Psychosocial support utilizing a team of specialists and social workers/counsellors, should be an integral part of pain management. Kvale E and Urba SG. National Comprehensive Cancer Network (NCCN) 19th Annual Conference, March 13 – 15, 2014; Hollywood, Florida
Patients may be able to better tolerate these agents if started at a lower dose and dose titrated slowly every three to five days. Peripheral neuropathic pain has been attributed to an unbalanced release of norepinephrine and serotonin from the neurons. Serotonin-Norepinephrine Reuptake Inhibitors (SNRI’s), including Venlafaxine (EFFEXOR®) and Duloxetine (CYMBALTA®), are better tolerated and have fewer drug interactions than TCA’s. EFFEXOR® in one study significantly relieved Oxaliplatin induced neuropathic pain in more than two thirds of the patients when compared to placebo and a third of the patients had complete pain relief. There is however limited evidence showing a beneficial role of Selective Serotonin Reuptake Inhibitors (SSRI’s) such as Citalopram (CELEXA®) and Paroxetine (PAXIL®) for neuropathic pain. As a note of caution, SNRI’s such as EFFEXOR® and CYMBALTA®, can interact with Tamoxifen, prescribed to patients with breast cancer, preventing Tamoxifen from converting to its active form. The dose of antidepressants needed to alleviate neuropathic pain is not dependent on antidepressant activity and may be lower than that recommended for treatment of depression. The newer anticonvulsants such as Gabapentin (NEURONTIN®), Pregabalin (LYRICA®) are preferable first line agents for the treatment of neuropathic pain rather than traditional, older agents such as Carbamazepine (TEGRETOL®), Phenytoin (DILANTIN®) and Valproate (DEPAKOTE®), as the newer agents are associated with fewer drug interactions. The newer agents bind to the alpha2-delta subunit of the calcium-sensitive channels, modulating neurotransmitter release. Of the newer agents, NEURONTIN® (Gabapentin) is not protein bound and is excreted unchanged in the urine and therefore has fewer drug interactions. If opioids are a consideration for neuropathic pain relief, the lowest dose is recommended. Topical therapies for neuropathic pain have the advantage of controlling pain without systemic side effects. It therefore can be combined with systemic treatment. Lidocaine 5% patches (LIDODERM®) block neuronal sodium channels whereas Capsaicin cream (ZOSTRIX®) stimulates the C fibers to release and subsequently deplete substance P, there by blocking pain signaling to the brain. Diclofenac gel 1% when applied once a day, concentrates in the dermis and has less gastrointestinal side effects and may be beneficial for neuropathic pain. A combination of Ketamine 1% and Amitriptyline 2% cream applied topically has also been promising in a small study. Patients experiencing refractory pain may benefit with the use of Transcutaneous Electrical Nerve Stimulation (TENS), although referral to the pain clinic may be appropriate. Psychosocial support utilizing a team of specialists and social workers/counsellors, should be an integral part of pain management. Kvale E and Urba SG. National Comprehensive Cancer Network (NCCN) 19th Annual Conference, March 13 – 15, 2014; Hollywood, Florida
The ASH Choosing Wisely® campaign five hematologic tests and treatments to question
SUMMARY: CHOOSING WISELY® is a quality improvement initiative led by the American Board of Internal Medicine Foundation in collaboration with leading medical societies in the United States such as the American Society of Hematology (ASH). This organization was established to improve quality of medical care, after it was noted that about 25% of the tests ordered at the time of hospital admission and 65% of the tests ordered on subsequent days were avoidable. Further, there is ample evidence to suggest that reducing unneeded investigations can decrease costs, increase patient satisfaction and quality of care. CHOOSING WISELY® has challenged medical societies to identify 5 tests, procedures or treatments, within each specialty's clinical domain, that are offered to patients, despite the lack of evidence demonstrating its benefit. The goal is to make positive changes in the actual delivery of patient care. The ASH identified 5 tests and treatments that practicing hematologists should give due consideration to, that in some situations are not evidence based and which in certain cases are associated with risks that outweigh the benefits and are not cost efficient.
Gaps in Pre-rituximab Hepatitis B Screening An Institutional Experience
SUMMARY:The Centers for Disease Control and Prevention (CDC) estimates that there are 800,000 -1.4 million individuals with Chronic Hepatitis B infection in the United States. Reactivation of HBV is a major concern in cancer patients who may be on chemotherapy or other immunosuppressive therapies, with the incidence of HBV reactivation ranging from 40%-60% in those who are positive for Hepatitis B surface antigen (HBsAg). HBV reactivation is preventable with prophylactic antiviral therapy, failing which it can result in delays in cancer treatment as well as potentially fatal outcomes. The CDC updated their recommendations in 2008 and recommended HBV screening for patients receiving cytotoxic chemotherapy or immunotherapy. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO) suggesting that there was insufficient evidence to recommend routine screening for HBV in cancer patients,but screening may be considered for patient populations at high risk or for those who are to receive highly immunosuppressive therapies including anti-CD20 monoclonal antibody therapy such as Rituximab (RITUXAN®). To evaluate compliance with these recommendations, the authors in this study retrospectively reviewed charts of patients with Low grade Non Hodgkins Lymphoma at a teritiary care center and documented the various studies performed, as a part of the pretreatment workup, between January 2005 and December 2011. They noted that only 19% of the total patients and 25% of the patients who received RITUXAN® had HBV screening done. The authors concluded that this was a significant deviation from the recommended guidelines and these findings resulted in the implementation of stricter measures for HBV screening at this teritiary care center. Screening for HBV should include testing for Hepatitis B surface antigen (HBsAg), Antibody to Hepatitis B core antigen (anti-HBc) and Antibody to Hepatitis B surface antigen (Anti-HBs). Patients positive for HBsAg and anti-HBc as well as those who are negative for HBsAg and positive for anti-HBc, should have testing for HBV viral load using serum HBV DNA and those without active disease should receive prophylactic antiviral therapy and be closely monitored for HBV reactivation. Prophylaxis is usually started one week before initiating chemotherapy and continued for at least 6 months after completion of chemotherapy, although the actual duration of prophylactic antiviral therapy remains unclear. If HBV reactivation is noted, chemotherapy should be immediately discontinued. Given the prevalence of chronic Hepatitis B in the United States, screening for HBV should become a routine part of pretreatment evaluation in cancer patients. Abbi KK, Gorris M, Skeel RT. Am J Ther. 2013;June 28.
The CDC updated their recommendations in 2008 and recommended HBV screening for patients receiving cytotoxic chemotherapy or immunotherapy. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO) suggesting that there was insufficient evidence to recommend routine screening for HBV in cancer patients,but screening may be considered for patient populations at high risk or for those who are to receive highly immunosuppressive therapies including anti-CD20 monoclonal antibody therapy such as Rituximab (RITUXAN®). To evaluate compliance with these recommendations, the authors in this study retrospectively reviewed charts of patients with Low grade Non Hodgkins Lymphoma at a teritiary care center and documented the various studies performed, as a part of the pretreatment workup, between January 2005 and December 2011. They noted that only 19% of the total patients and 25% of the patients who received RITUXAN® had HBV screening done. The authors concluded that this was a significant deviation from the recommended guidelines and these findings resulted in the implementation of stricter measures for HBV screening at this teritiary care center. Screening for HBV should include testing for Hepatitis B surface antigen (HBsAg), Antibody to Hepatitis B core antigen (anti-HBc) and Antibody to Hepatitis B surface antigen (Anti-HBs). Patients positive for HBsAg and anti-HBc as well as those who are negative for HBsAg and positive for anti-HBc, should have testing for HBV viral load using serum HBV DNA and those without active disease should receive prophylactic antiviral therapy and be closely monitored for HBV reactivation. Prophylaxis is usually started one week before initiating chemotherapy and continued for at least 6 months after completion of chemotherapy, although the actual duration of prophylactic antiviral therapy remains unclear. If HBV reactivation is noted, chemotherapy should be immediately discontinued. Given the prevalence of chronic Hepatitis B in the United States, screening for HBV should become a routine part of pretreatment evaluation in cancer patients. Abbi KK, Gorris M, Skeel RT. Am J Ther. 2013;June 28.
SYLVANT® (Siltuximab)
The FDA on April 23, 2014 approved SYLVANT® for the treatment of patients with multicentric Castleman’s disease (MCD) who are human immunodeficiency virus (HIV-) -negative and human herpes virus -8 (HHV-8) -negative. SYLVANT® injection is a product of Janssen Biotech, Inc.
The Role of Human Papillomavirus in Nongenital Cancers
SUMMARY: Human Papilloma Virus (HPV) is a double stranded DNA virus and is the most common sexually transmitted infection in the U.S. It was responsible for over 25,000 cancers between 2004 and 2007 in the U.S. and the incidence is rapidly increasing due to changes in sexual practices. Even though the low risk HPV types such as HPV-6 and HPV-11 have been well known to cause benign lesions such as condylomata (genital warts), low grade squamous intraepithelial lesions of the cervix and laryngeal papillomas, the high risk HPV types such as HPV-16 and HPV-18 have been of major concern because of their malignant potential. Since the implication of HPV-16 and HPV-18 in cervical cancer dating back to the early 1990’s, these HPV subtypes have also been found responsible for 45-90% of oropharyngeal cancers and 90% of anal cancers. HPV in tumor tissue can be detected by immunohistochemistry testing for P16 expression and confirmed with HPV DNA PCR. 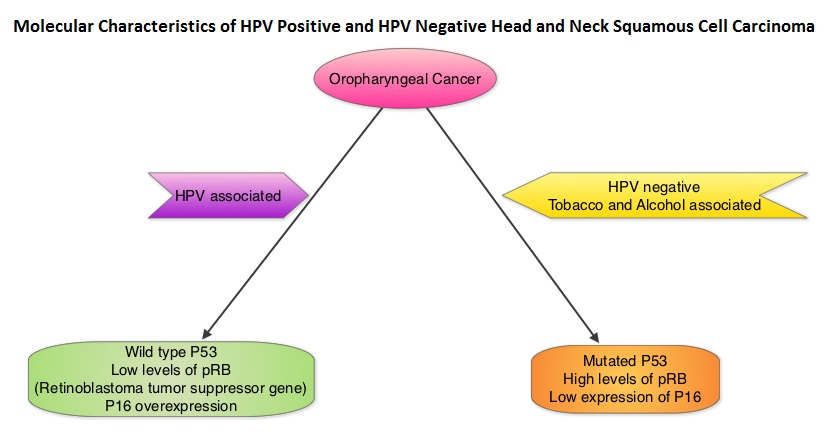 Chronic immunosuppression as seen in patients with HIV and in patients undergoing solid organ transplantation, may increase the risk for HPV infections. Patients with HPV associated oropharyngeal cancer typically are younger males, tend not to smoke or drink and present with poorly differentiated, non keratinizing tumors with basaloid morphology, compared to those with HPV negative tumors. Clinical characteristics of HPV positive oropharyngeal cancer patients with best outcomes include, those with fewer than 10 pack year smoking history and lower tumor stage. Several retrospective trials as well as some small prospective studies have shown that HPV positive oropharygeal cancers when treated with chemoradiation have significantly higher response rates, progression free survival, overall survival and better local and regional disease control. In the TAX 324 randomized phase III trial, patients received induction treatment with 3 cycles of TAXOTERE®, Cisplatin and 5-Fluorouracil (5-FU) or Cisplatin and 5-FU followed by chemoradiation with concurrent PARAPLATIN® (Carboplatin). Even though the 3 drug induction treatment group had superior outcomes compared to those who received 2 drug induction regimen in the intent to treat population, on retrospective analysis, patients with HPV positive oropharyngeal cancer had a significantly longer 5 year progression free survival (78% vs 28%) and overall survival, with an 80% reduction in mortality (HR=0.20, P<0.0001), compared to HPV negative patients, regardless of induction treatment. Other studies have shown that HPV positive patients who undergo surgery alone for oropharyngeal cancer do not appear to reap these favorable benefits, suggesting that the improved prognosis in the HPV positive patients with oropharyngeal cancer is related to chemotherapy and radiation. It also appears that HPV positive patients with oropharyngeal cancer have a better prognosis with treatment when their tumors are P53 wild type and express P16. With regards to EGFR and P16, there appears to be an inverse correlation between P16 and EGFR expression and patients with tumors expressing P16 and not EGFR have a significantly higher 5 year disease free and overall survival compared to those whose tumors overexpress EGFR but not P16. This information may have significant therapeutic implications and studies are underway trying to address this group of patients with targeted and less intense treatments. It should be noted that HPV positive status has a favorable prognostic value only for oropharyngeal primary cancers and not for other cancers of the head and neck.
Chronic immunosuppression as seen in patients with HIV and in patients undergoing solid organ transplantation, may increase the risk for HPV infections. Patients with HPV associated oropharyngeal cancer typically are younger males, tend not to smoke or drink and present with poorly differentiated, non keratinizing tumors with basaloid morphology, compared to those with HPV negative tumors. Clinical characteristics of HPV positive oropharyngeal cancer patients with best outcomes include, those with fewer than 10 pack year smoking history and lower tumor stage. Several retrospective trials as well as some small prospective studies have shown that HPV positive oropharygeal cancers when treated with chemoradiation have significantly higher response rates, progression free survival, overall survival and better local and regional disease control. In the TAX 324 randomized phase III trial, patients received induction treatment with 3 cycles of TAXOTERE®, Cisplatin and 5-Fluorouracil (5-FU) or Cisplatin and 5-FU followed by chemoradiation with concurrent PARAPLATIN® (Carboplatin). Even though the 3 drug induction treatment group had superior outcomes compared to those who received 2 drug induction regimen in the intent to treat population, on retrospective analysis, patients with HPV positive oropharyngeal cancer had a significantly longer 5 year progression free survival (78% vs 28%) and overall survival, with an 80% reduction in mortality (HR=0.20, P<0.0001), compared to HPV negative patients, regardless of induction treatment. Other studies have shown that HPV positive patients who undergo surgery alone for oropharyngeal cancer do not appear to reap these favorable benefits, suggesting that the improved prognosis in the HPV positive patients with oropharyngeal cancer is related to chemotherapy and radiation. It also appears that HPV positive patients with oropharyngeal cancer have a better prognosis with treatment when their tumors are P53 wild type and express P16. With regards to EGFR and P16, there appears to be an inverse correlation between P16 and EGFR expression and patients with tumors expressing P16 and not EGFR have a significantly higher 5 year disease free and overall survival compared to those whose tumors overexpress EGFR but not P16. This information may have significant therapeutic implications and studies are underway trying to address this group of patients with targeted and less intense treatments. It should be noted that HPV positive status has a favorable prognostic value only for oropharyngeal primary cancers and not for other cancers of the head and neck.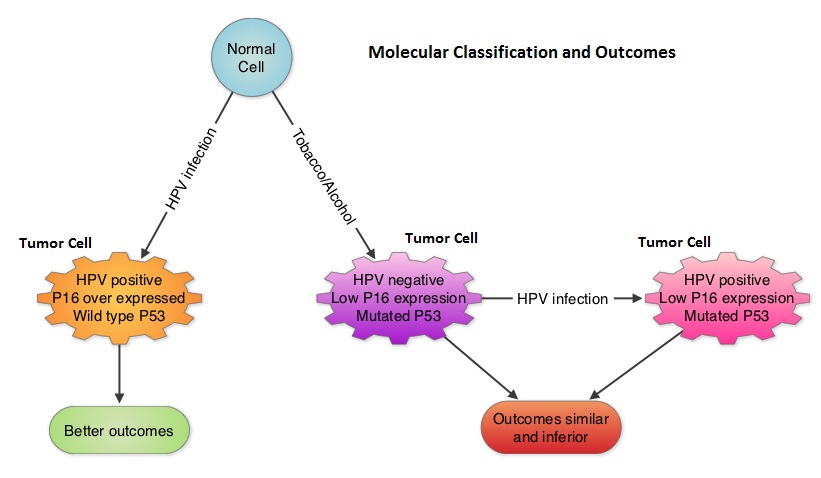
With regards to anal carcinoma, there appears to be a relationship between cervical, anogenital and oropharyngeal cancer suggesting a genital-anal-oral transmission of HPV. Patients with HIV infection have a higher risk of developing HPV associated anal carcinoma and antiretroviral therapy does not decrease this risk. Anal Pap test is recommended annually for high risk patients including those with a history of anogental warts and women with abnormal cervical or vulvar cytology. For patients with anal carcinoma, positive HPV status does not confer a favorable prognosis as is the case for patients with oropharygeal carcinoma. GARDASIL®, a quadrivalent vaccine targeting HPV-6,11,16 and 18 as well as CERVARIX®, a bivalent vaccine targeting HPV-16 and 18 are presently available in the U.S. They are recommended for both females and males at an age as early as 9 years and given as a 3 shot series, to prevent HPV related Cervical Intraepithelial lesions/cervical cancer and genital warts/Anal Intraepithelial Neoplasia respectively. The authors conclude that HPV infection and associated malignancies are preventable and attempts should be made to eradicate this virus. Zandberg DP, Bhargava R, Badin S, et al. CA Cancer J Clin 2013;63:57-81
Impact of More Restrictive Blood Transfusion Strategies on Clinical Outcomes A Meta-analysis and Systematic Review
SUMMARY: The traditional hemoglobin trigger to recommend blood transfusions for majority of the Health Care Providers is between 7.5 and 9 g/dl. The clinical rationale is based on the premise that increasing Hgb levels increases blood oxygen content and possible oxygen delivery to the tissues. However, there are no randomized trials validating improved oxygen delivery to tissues or better clinical outcomes in any setting at this hemoglobin transfusion trigger. The authors in this provocative study conducted a comprehensive research and performed a Primary and Secondary meta-analysis. In their primary meta-analysis, they reviewed the pooled data from 3 randomized clinical trials with 2364 patients and in these trials, a less than 7g/dl hemoglobin as a transfusion trigger (restrictive transfusion strategy) was compared with a more liberal transfusion strategy and outcomes were evaluated. These endpoints included mortality, acute coronary syndrome, pulmonary edema, infections and re-bleeding risk. The combined data from these 3 trials showed that a restrictive transfusion strategy resulted in a 26% mortality reduction in hospitalized patients, 20% reduction in total mortality, 36% reduction in the risk of re-bleeding, 56% reduction in acute coronary syndrome, 52% reduction in the incidence of pulmonary edema and 14% reduction in bacterial infections, compared with a more liberal transfusion strategy. The secondary meta-analysis evaluated patients in 16 trials (these were excluded from the primary meta-analysis) that used a less restrictive transfusion trigger (hemoglobin transfusion triggers of 7.5-10g/dl) and the authors noted that outcomes were not improved with a more liberal transfusion strategy. Further it was also noted that several observational studies have shown that Hgb levels of 5-6g/dl was well tolerated in normovolemic patients without effecting oxygen delivery. Contrary to clinical presumptions, these counter-intuitive findings can be explained based on sound physiologic principles. Normovolemic hemodilution following administration of crystalloid or colloid solutions, to replace blood loss, has been associated with a reduction in systemic vascular resistance, increase in cardiac output, coronary and cerebral blood flow and synthesis of 2,3-diphosphoglycerate in red blood cells thus maintaining movement of oxygen from red blood cells to body tissues. Liberal blood transfusions may in fact impair oxygen uptake by vital tissues by increasing the blood viscosity and the resulting loss of RBC function during preservation and storage of blood. Studies have also shown that in patients with gastrointestinal bleeding, restrictive transfusion strategy results in a lower portal blood pressure and less recurrent bleeding, as higher blood pressures might disrupt a thrombus plug. The authors following this clinically relevant meta-analysis concluded that restrictive transfusion strategy resulted in better outcomes and transfusion triggers should be evidence based. Salpeter SR, Buckley JS and Chatterjee S. The American Journal of Medicine 2014;127:124-131
