Written by: AJMC® Editorial Staff
Content Sponsored by: BeiGene
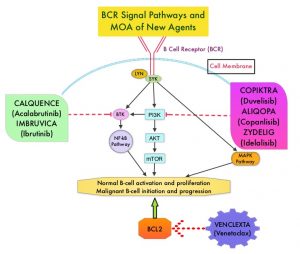
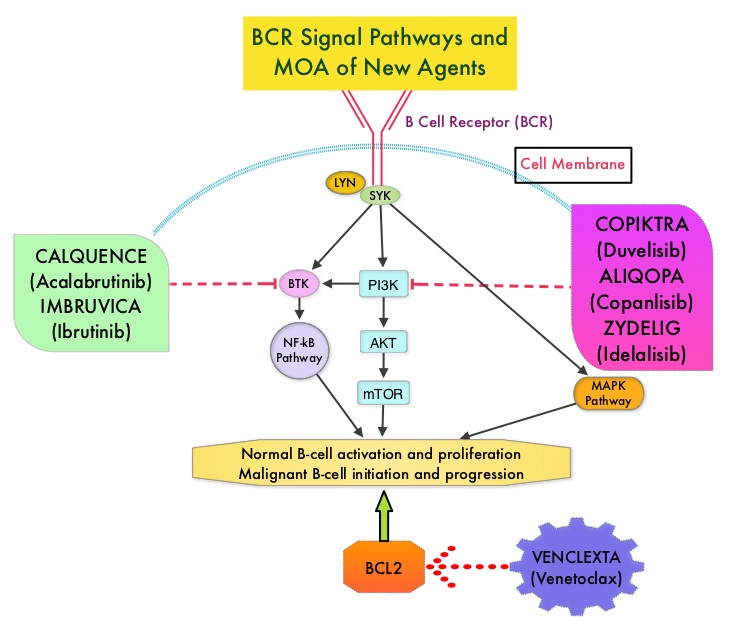
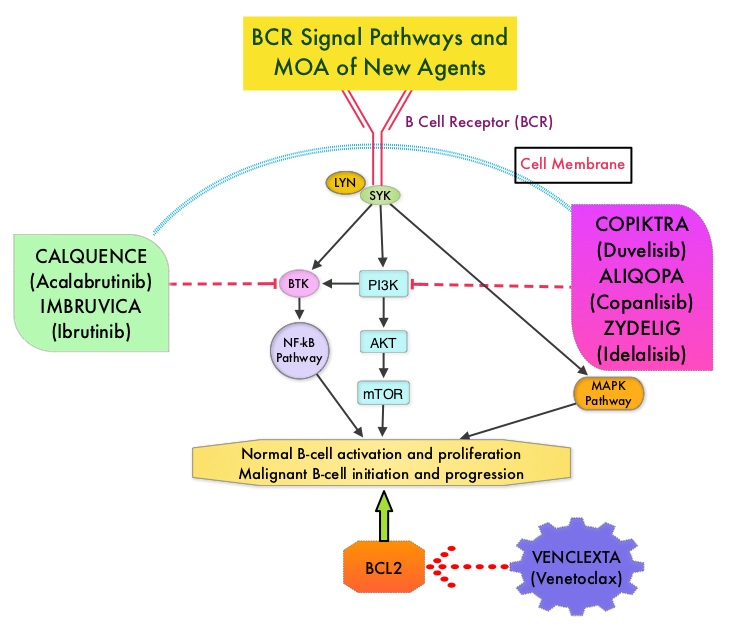
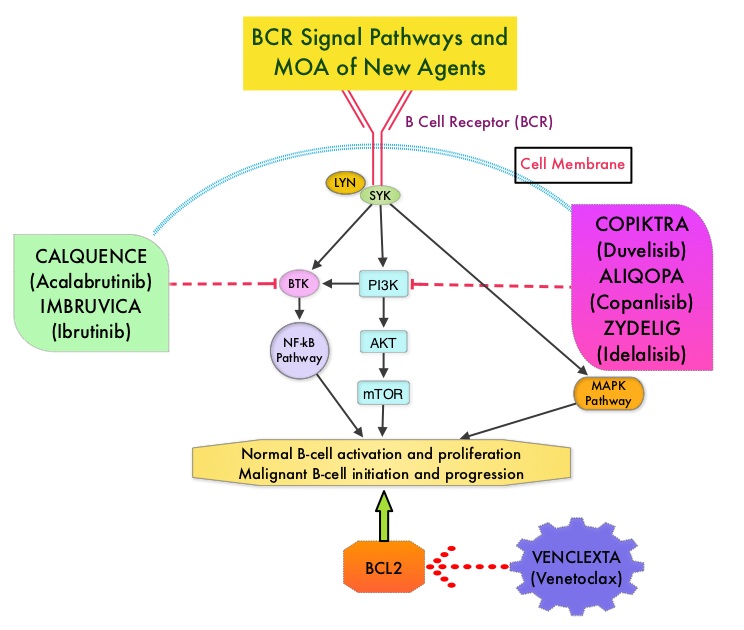
Adults experience chronic lymphocytic leukemia (CLL) at a greater rate than they do any other type of leukemia.1 In 2014, ibrutinib became the first Bruton tyrosine kinase (BTK) inhibitor approved by the FDA for the treatment of CLL.2,3 In a 3-year safety study of patients with CLL/small lymphocytic lymphoma (SLL) taking a daily dose of this first-generation medication, ibrutinib was shown to have both a high response rate that increased in quality and frequency over time and modest toxicity.4
However, treatment with ibrutinib has been shown to be associated with adverse events (AEs) that can lead to discontinuation and/or down-dosing.5,6 In particular, atrial fibrillation (AF) is a known AE associated with BTK inhibitor treatment that has been reported in clinical trials.7,8
To determine the economic burden of down-dosing and therapy discontinuation due to AEs after initiation of ibrutinib therapy in patients with CLL/SLL, a team from Milliman, Inc, analyzed 2015 to 2019 data from a proprietary Medicare Advantage claims database that contains annual enrollment information and all Parts A, B, and D claims for approximately 2.5 million annual members.9 Investigators identified patients who developed AF within the first 12 months of starting treatment with ibrutinib. The results of this claims-based analysis were presented during a Science & Innovation Theater presented during the Academy of Managed Care Pharmacy Nexus 2021, held from October 18 to 21, 2021, in Denver, Colorado.9
In the group identified for analysis, investigators examined rates of and average time to discontinuation, down-dosing, and AEs as well as total health care costs accumulated during the 12 months following ibrutinib start.9 Using these key metrics, investigators then compared patients who newly experienced AF (new AF) during this 12-month episode period with those who did not experience new AF during this period. The results of the analysis showed that patients with claims for new AF discontinued ibrutinib at more than twice the rate of patients without claims for new AF and had significantly higher health care utilization.9 These results are explored in detail in a review article, “Real-World Evidence Associated with a First-Generation BTK Inhibitor in Patients With CLL/SLL,” published by The American Journal of Managed Care® (AJMC®) on ajmc.com. In an interview following the review, principal investigator and health care consultant from Milliman, Inc, Kathryn Fitch, RN, MEd, discusses the study’s findings regarding treatment patterns among patients who developed new AF after starting ibrutinib, the costs associated with the development of new AF, and her team’s recent research in this field. In a final interview, Michael Kolodziej, MD, FACP, medical oncologist and Senior Advisor at ADVI Health, LLC, discussed the analysis and its potential implications for managed care.
REFERENCES
1. The American Cancer Society Medical and Editorial Content Team. What is chronic lymphocytic leukemia? American Cancer Society. Updated May 10, 2018. January 13, 2022. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/about/what-is-cll.html
2. Chronic lymphocytic leukemia/small lymphocytic lymphoma: FDA updates. Lymphoma Research Foundation. Updated April 21, 2020. Accessed January 13, 2022. https://lymphoma.org/aboutlymphoma/cll/cllfdaupdates/
3. Center for Drug Evaluation and Research. Approval package for application number 205552Orig2s000. Trade name: Imbruvica. United States Food and Drug Administration. February 12, 2014. Accessed January 13, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205552Orig2s000Approv.pdf
4. Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497-2506. doi:10.1182/blood-2014-10-606038
5. Imbruvica. Prescribing information. Janssen Biotech; 2020. Accessed January 13, 2022. https://www.imbruvica.com/files/prescribing-information.pdf
6. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874-879. doi:10.3324/haematol.2017.182907
7. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805. doi:10.3324/haematol.2017.171041
8. Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14(2):e0211228. doi:10.1371/journal.pone.0211228
9. Fitch KV. Assessing the treatment emergent burden in BTKi therapy: a Medicare analysis in CLL (chronic lymphocytic leukemia). Presented at the Academy of Managed Care Pharmacy Nexus 2021; October 20, 2021; Denver, CO. Accessed January 18, 2022. https://2021.amcpnexus.org/program/science-innovation-theaters