SUMMARY: The FDA on September 30, 2013 granted accelerated approval to PERJETA® (Pertuzumab) for use in combination with HERCEPTIN® (Trastuzumab) and other chemotherapy for the neoadjuvant (preoperative) treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or with positive lymph nodes), as part of a complete treatment regimen for early breast cancer. Following surgery, patients should continue to receive HERCEPTIN® to complete one year of treatment. The HER family of receptors consist of HER1, HER2, HER3 and HER4.  These proteins are transmembrane tyrosine kinases and are involved in normal cell growth and differentiation. HER1 is also known as Epidermal Growth Factor Receptor or EGFR. These receptors are activated following ligand binding, receptor pairing or dimerization and phosphorylation. This dimerization (receptor pairing) occurs often within the HER family of receptors. This has been no ligand identified for HER2 receptor, although it is able to form homo and heterodimers with other members of the HER family readily. Dimerization of HER2 and HER3 is believed to produce the strongest mitogenic signaling and activates two important pathways that regulate cell survival and growth – Mitogen Activated Protein Kinase (MAPK) pathway and PhosphoInositide 3-Kinase (PI3K) pathway. For this reason inhibiting HER2 dimerization appears to be an important step in the treatment of cancer. Overexpression of HER2 in breast cancer has been associated with higher risk for relapse as well as overall survival. Approximately 20 percent of breast cancers are HER2-positive. HERCEPTIN® is a humanized monoclonal antibody targeting HER2. It binds to the extracellular subdomain IV of the receptor and disrupts ligand independent HER2 downstream cell signaling pathways. PERJETA® is a recombinant, humanized, monoclonal antibody that binds to the HER2 subdomain II and blocks ligand dependent HER2 heterodimerization with other HER receptors, ie. HER3, HER1 and HER4. Thus HERCEPTIN® along with PERJETA® provide a more comprehensive blockade of HER2 driven signaling pathways. The accelerated approval of PERJETA® for the neoadjuvant treatment of breast cancer was based on a randomized, multicenter, open-label, phase II trial, in which 417 patients with HER2-positive, operable, locally advanced or inflammatory breast cancer (T2-4d), were randomly assigned to receive preoperative therapy with either HERCEPTIN® plus TAXOTERE® (Docetaxel), PERJETA® plus HERCEPTIN® and TAXOTERE®, PERJETA® plus HERCEPTIN® or PERJETA® plus TAXOTERE®. Patients in the three drug group received preoperative therapy with PERJETA®, HERCEPTIN® and TAXOTERE® every 3 weeks for a total of 4 cycles and following surgery, all patients received 3 cycles of Fluorouracil, ELLENCE® (Epirubicin), and CYTOXAN® (Cyclophosphamide) – (FEC) IV every 3 weeks and HERCEPTIN® was continued every 3 weeks for a total of one year of therapy. The primary endpoint was pathological Complete Response (pCR) rate defined as the absence of invasive cancer in the breast. The FDA definition of pCR is the absence of invasive cancer in the breast and lymph nodes. All treatment groups were well balanced. Seven percent of patients had inflammatory breast cancer, 32% had locally advanced cancer and 70% had clinically node-positive breast cancer. Forty-seven percent of the patients had hormone receptor-positive disease. The FDA defined pCR rates were 39.3% in the PERJETA® plus HERCEPTIN® and TAXOTERE® group and 21.5% in the HERCEPTIN® plus TAXOTERE® group (P=0.0063). Of Interest, the pCR rates in the three drug group were lower in patients with hormone receptor positive tumors compared to patients with hormone receptor negative tumors. The most common adverse events in the three drug group were alopecia, diarrhea, nausea and neutropenia. Other significant side effects included decreased cardiac function, infusion-related reactions, hypersensitivity reactions and anaphylaxis. Based on clinical studies, for the neoadjuvant treatment of breast cancer, PERJETA® should be administered every 3 weeks for 3 to 6 cycles as part of one of the following treatment regimens for early breast cancer. • Four preoperative cycles of PERJETA® in combination with HERCEPTIN® and TAXOTERE® followed by 3 postoperative cycles of Fluorouracil, ELLENCE® and CYTOXAN® (FEC). • Three preoperative cycles of FEC alone followed by 3 preoperative cycles of PERJETA® in combination with TAXOTERE® and HERCEPTIN®. • Six preoperative cycles of PERJETA® in combination with TAXOTERE®, Carboplatin, and HERCEPTIN® (TCH). Following surgery, patients should continue to receive HERCEPTIN® to complete 1 year of treatment. The accelerated approval by the FDA was based solely on the improved pCR rate with the three drug combination with no demonstrable improvement in event-free survival or overall survival. A confirmatory phase III trial is underway, with results expected in 2016. Gianni L, Pienkowski T, Im YH, et al. Lancet Oncol. 2012;13:25-32
These proteins are transmembrane tyrosine kinases and are involved in normal cell growth and differentiation. HER1 is also known as Epidermal Growth Factor Receptor or EGFR. These receptors are activated following ligand binding, receptor pairing or dimerization and phosphorylation. This dimerization (receptor pairing) occurs often within the HER family of receptors. This has been no ligand identified for HER2 receptor, although it is able to form homo and heterodimers with other members of the HER family readily. Dimerization of HER2 and HER3 is believed to produce the strongest mitogenic signaling and activates two important pathways that regulate cell survival and growth – Mitogen Activated Protein Kinase (MAPK) pathway and PhosphoInositide 3-Kinase (PI3K) pathway. For this reason inhibiting HER2 dimerization appears to be an important step in the treatment of cancer. Overexpression of HER2 in breast cancer has been associated with higher risk for relapse as well as overall survival. Approximately 20 percent of breast cancers are HER2-positive. HERCEPTIN® is a humanized monoclonal antibody targeting HER2. It binds to the extracellular subdomain IV of the receptor and disrupts ligand independent HER2 downstream cell signaling pathways. PERJETA® is a recombinant, humanized, monoclonal antibody that binds to the HER2 subdomain II and blocks ligand dependent HER2 heterodimerization with other HER receptors, ie. HER3, HER1 and HER4. Thus HERCEPTIN® along with PERJETA® provide a more comprehensive blockade of HER2 driven signaling pathways. The accelerated approval of PERJETA® for the neoadjuvant treatment of breast cancer was based on a randomized, multicenter, open-label, phase II trial, in which 417 patients with HER2-positive, operable, locally advanced or inflammatory breast cancer (T2-4d), were randomly assigned to receive preoperative therapy with either HERCEPTIN® plus TAXOTERE® (Docetaxel), PERJETA® plus HERCEPTIN® and TAXOTERE®, PERJETA® plus HERCEPTIN® or PERJETA® plus TAXOTERE®. Patients in the three drug group received preoperative therapy with PERJETA®, HERCEPTIN® and TAXOTERE® every 3 weeks for a total of 4 cycles and following surgery, all patients received 3 cycles of Fluorouracil, ELLENCE® (Epirubicin), and CYTOXAN® (Cyclophosphamide) – (FEC) IV every 3 weeks and HERCEPTIN® was continued every 3 weeks for a total of one year of therapy. The primary endpoint was pathological Complete Response (pCR) rate defined as the absence of invasive cancer in the breast. The FDA definition of pCR is the absence of invasive cancer in the breast and lymph nodes. All treatment groups were well balanced. Seven percent of patients had inflammatory breast cancer, 32% had locally advanced cancer and 70% had clinically node-positive breast cancer. Forty-seven percent of the patients had hormone receptor-positive disease. The FDA defined pCR rates were 39.3% in the PERJETA® plus HERCEPTIN® and TAXOTERE® group and 21.5% in the HERCEPTIN® plus TAXOTERE® group (P=0.0063). Of Interest, the pCR rates in the three drug group were lower in patients with hormone receptor positive tumors compared to patients with hormone receptor negative tumors. The most common adverse events in the three drug group were alopecia, diarrhea, nausea and neutropenia. Other significant side effects included decreased cardiac function, infusion-related reactions, hypersensitivity reactions and anaphylaxis. Based on clinical studies, for the neoadjuvant treatment of breast cancer, PERJETA® should be administered every 3 weeks for 3 to 6 cycles as part of one of the following treatment regimens for early breast cancer. • Four preoperative cycles of PERJETA® in combination with HERCEPTIN® and TAXOTERE® followed by 3 postoperative cycles of Fluorouracil, ELLENCE® and CYTOXAN® (FEC). • Three preoperative cycles of FEC alone followed by 3 preoperative cycles of PERJETA® in combination with TAXOTERE® and HERCEPTIN®. • Six preoperative cycles of PERJETA® in combination with TAXOTERE®, Carboplatin, and HERCEPTIN® (TCH). Following surgery, patients should continue to receive HERCEPTIN® to complete 1 year of treatment. The accelerated approval by the FDA was based solely on the improved pCR rate with the three drug combination with no demonstrable improvement in event-free survival or overall survival. A confirmatory phase III trial is underway, with results expected in 2016. Gianni L, Pienkowski T, Im YH, et al. Lancet Oncol. 2012;13:25-32
Tag: Breast Cancer
Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer a meta-analysis of individual patient data from randomised trials
SUMMARY: Bisphosphonates are presently indicated for the treatment of osteoporosis. The approved bisphosphonates in the U.S. include FOSAMAX® (Alendronate), BONIVA® (Ibandronate), ACTONEL® (Risedronate) and RECLAST® (Zoledronic acid). Several trials conducted over the past 2 decades have suggested that bisphosphonates may have anti proliferative effect in patients with breast cancer. Data from several Women's Health Initiative (WHI) studies involving more than 150,000 healthy postmenopausal women, of whom 2216 used oral bisphosphonates, revealed that women taking bisphosphonates for osteoporosis had a 32% reduction in invasive breast cancer.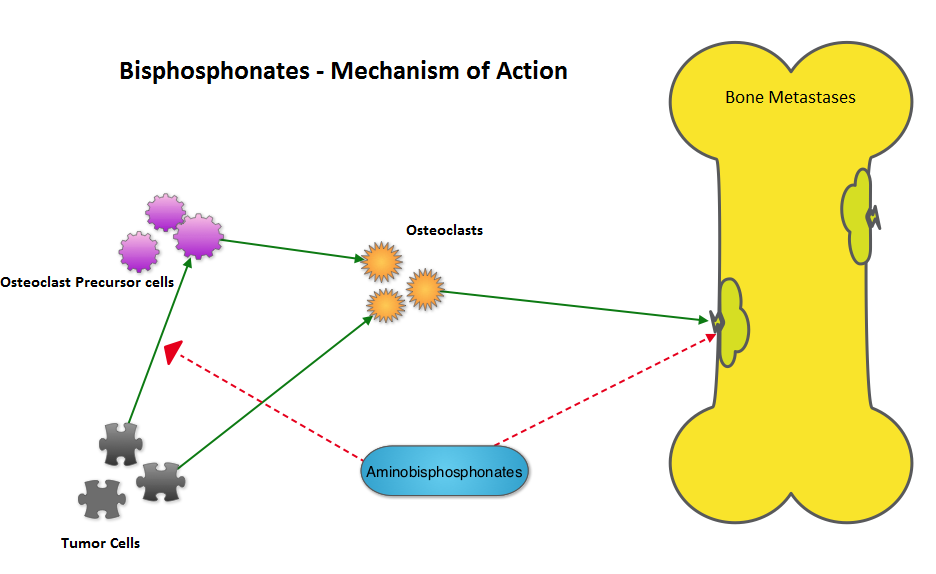 The AZURE investigators conducted a study to determine whether the addition of RECLAST® (Zoledronic acid) to standard adjuvant therapy would improve disease outcomes in patients with early-stage breast cancer. They noted that in the subset analysis, the addition of RECLAST® significantly improved disease free survival and overall survival in postmenopausal patients, independent of estrogen receptor status, tumor stage, and lymph node involvement (N Engl J Med 2011;365:1396-1405). With this background, the authors belonging to the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), conducted a meta-analysis and reviewed data from 15 years of bisphosphonate trials, which included 36 trials of adjuvant bisphosphonates in breast cancer and involved over 17,000 pre and postmenopausal women. RECLAST® (Zoledronic acid) and Clodronate were the most common bisphosphonates used in these trials. The primary outcomes analyzed were time to distant recurrence, local recurrence, new second primary breast cancer (ipsilateral or contralateral), time to first distant recurrence (ignoring any previous locoregional or contralateral recurrences), and breast cancer mortality. Planned subset analyses included site of recurrence, site of first distant metastasis (bone vs other), menopausal status (pre, peri and post) type of bisphosphonate (aminobisphosphonates such as RECLAST® or Clodronate) and drug schedule of bisphosphonate therapy (for bone protection vs advanced cancer). Adjuvant bisphosphonates resulted in a 34% reduction in the risk of bone recurrence (P = 0.00001) and a 17% reduction in the risk of breast cancer death (P =0.004). This benefit was seen regardless of estrogen receptor status, nodal status or whether chemotherapy was used or not. Bisphosphonates had no significant impact on non-breast cancer related deaths, contralateral breast cancer or loco-regional recurrence. In this meta-analysis, all these benefits were only seen in postmenopausal women and premenopausal women had no benefit on any disease outcomes with bisphosphonates. The authors emphasized that low estrogen environment as is seen in postmenopausal women, or women rendered menopausal by suppression of ovarian function is a prerequisite for adjuvant bisphosphonate activity. Based on this large meta-analysis, the authors recommended the use of RECLAST® once every six months or oral Clodronate, where available. Because of paucity of data, they do not recommend the use of weekly dose of oral bisphosphonates, often used to prevent osteoporosis, to achieve these benefits. Coleman R, Gnant M, Paterson A, et al. San Antonio Breast Cancer Symposium 2013; San Antonio, TX. Abstract S4-07.
The AZURE investigators conducted a study to determine whether the addition of RECLAST® (Zoledronic acid) to standard adjuvant therapy would improve disease outcomes in patients with early-stage breast cancer. They noted that in the subset analysis, the addition of RECLAST® significantly improved disease free survival and overall survival in postmenopausal patients, independent of estrogen receptor status, tumor stage, and lymph node involvement (N Engl J Med 2011;365:1396-1405). With this background, the authors belonging to the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), conducted a meta-analysis and reviewed data from 15 years of bisphosphonate trials, which included 36 trials of adjuvant bisphosphonates in breast cancer and involved over 17,000 pre and postmenopausal women. RECLAST® (Zoledronic acid) and Clodronate were the most common bisphosphonates used in these trials. The primary outcomes analyzed were time to distant recurrence, local recurrence, new second primary breast cancer (ipsilateral or contralateral), time to first distant recurrence (ignoring any previous locoregional or contralateral recurrences), and breast cancer mortality. Planned subset analyses included site of recurrence, site of first distant metastasis (bone vs other), menopausal status (pre, peri and post) type of bisphosphonate (aminobisphosphonates such as RECLAST® or Clodronate) and drug schedule of bisphosphonate therapy (for bone protection vs advanced cancer). Adjuvant bisphosphonates resulted in a 34% reduction in the risk of bone recurrence (P = 0.00001) and a 17% reduction in the risk of breast cancer death (P =0.004). This benefit was seen regardless of estrogen receptor status, nodal status or whether chemotherapy was used or not. Bisphosphonates had no significant impact on non-breast cancer related deaths, contralateral breast cancer or loco-regional recurrence. In this meta-analysis, all these benefits were only seen in postmenopausal women and premenopausal women had no benefit on any disease outcomes with bisphosphonates. The authors emphasized that low estrogen environment as is seen in postmenopausal women, or women rendered menopausal by suppression of ovarian function is a prerequisite for adjuvant bisphosphonate activity. Based on this large meta-analysis, the authors recommended the use of RECLAST® once every six months or oral Clodronate, where available. Because of paucity of data, they do not recommend the use of weekly dose of oral bisphosphonates, often used to prevent osteoporosis, to achieve these benefits. Coleman R, Gnant M, Paterson A, et al. San Antonio Breast Cancer Symposium 2013; San Antonio, TX. Abstract S4-07.
Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II) an international, double-blind, randomised placebo-controlled trial
SUMMARY: NOLVADEX® (Tamoxifen) is a Selective Estrogen Receptor Modulator (SERM), approved by the FDA to also reduce the incidence of breast cancer in women considered to be at high risk (ChemoPrevention). This agent however has been linked to endometrial cancer and thromboembolic phenomenon in some women. ARIMIDEX® is a non-steroidal Aromatase Inhibitor proven to be more efficacious than NOLVADEX® both in metastatic and adjuvant settings. Similar to NOLVADEX®, ARIMIDEX® also prevents the occurrence of new primary tumors in the contralateral breast, in postmenopausal females. With this background, the International Breast Cancer Intervention Study (IBIS) -II trial enrolled 3864 postmenopausal women considered to be at increased risk of breast cancer and randomized them to receive either ARIMIDEX® (Anastrazole) 1 mg QD (N=1920) or Placebo QD (N=1944)for 5 years. Patients were considered to be at high risk if they had a family history of breast cancer, atypical ductal hyperplasia, lobular carcinoma in-situ or dense breast tissue. The median age was 59 years. The primary end point was histologically confirmed Invasive breast cancer or Ductal Carcinoma In- Situ. At a median follow up of 5 years, the incidence of breast cancer in the ARIMIDEX® group was 2% and in the placebo group was 4%. The predicted cumulative incidence of breast cancer after 7 years was 2.8% in the ARIMIDEX® group and 5.6% in the placebo group (HR=0.47; P<0.0001). This represented a 53% reduction in the risk of breast cancer. The adverse events were comparable in both groups with slight increase in the musculoskeletal and vasomotor events noted in the ARIMIDEX® group. The authors concluded that ARIMIDEX® reduced the risk of primary breast cancer by more than 50% in high risk postmenopausal women. Other unrelated studies have shown that acceptance of breast cancer prevention with medications (ChemoPrevention) appeared to be related to education and income, putting emphasis on education and adequate counseling of women, considered to be at high risk. Cuzick J, Sestak I, Forbes JF, et al. The Lancet, Early Online Publication, 12 December 2013
Safety and Efficacy of Everolimus With Exemestane vs Exemestane Alone in Elderly Patients With HER2-Negative, Hormone Receptor-Positive Breast Cancer in BOLERO-2
SUMMARY: Exemestane (AROMASIN®) is a steroidal, Aromatase Inhibitor (AI) which has been shown to improve Progression Free Survival (PFS) in postmenopausal patients with ER positive metastatic breast cancer. Patients tend to have limited clinical benefit with subsequent hormonal interventions if their disease recurs or progresses on AI’s. Further, cytotoxic chemotherapy may not be feasible in elderly patients. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. In the phase III BOLERO-2 study, patients with ER positive, advanced breast cancer, who had recurred or progressed on prior therapy with nonsteroidal AI’s such as Anastrozole (ARIMIDEX®) or Letrazole (FEMARA®), had doubling of PFS with a combination of AROMASIN® and AFINITOR® compared to AROMASIN® alone. The authors now report the Safety and Efficacy of AROMASIN® and AFINITOR® combination in 316 patients, 65 years of age or older, out of the 724 patients in the BOLERO-2 study. The median follow up was 18 months. Baseline treatment characteristics were similar in all treatment subsets. The treatment combination resulted in 55% reduction in the risk of disease progression regardless of the age compared to AROMASIN® alone (PFS – 7.8 months vs 3.2 months, HR=0.45, P<0.0001). Further the combination treatment resulted in greater clinical benefit compared to single agent AROMASIN®. Approximately two third of the patients regardless of their age required dose modifications and the most common adverse events in all age groups with similar incidences were stomatitis, rash, fatigue , hyperglycemia and pneumonitis. It is recommended that elderly patients be carefully monitored and toxicities promptly addressed during treatment. The authors concluded that a combination of AROMASIN® and AFINITOR® can provide significant clinical benefit with acceptable toxicities, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Pritchard KI, Burris HA, Ito Y, et al. Clinical Breast Cancer 2013;13:421-432.
PERJETA® combination Improves Response Rates
The FDA on September 30, 2013 approved PERJETA® for use in combination with HERCEPTIN® (Trastuzumab) and TAXOTERE® (Docetaxel) for the neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer. The accelerated approval by the FDA was based solely on the improved pCR rate with the three drug combination with no demonstrable improvement in event-free survival or overall survival. A confirmatory phase III trial is underway, with results expected in 2016. One would hope that the complete response rates would translate into improved survival. Stay tuned.
Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere) a randomised multicentre, open-label, phase 2 trial
SUMMARY: PERJETA® (Pertuzumab) is a recombinant, humanized, monoclonal antibody that binds to the HER2 dimerization domain and prevents the dimerization of HER2 with other HER receptors, ie. HER3, HER1 and HER4. HERCEPTIN® (Trastuzumab) is a monoclonal antibody that specifically targets the HER2 receptor and blocks the downstream signalling pathways. The accelerated approval of PERJETA® for the neoadjuvant treatment of breast cancer was based on a randomized, multicenter, open-label, phase II trial, in which 417 patients with HER2-positive, operable, locally advanced or inflammatory breast cancer (T2-4d), were randomly assigned to receive preoperative therapy with either HERCEPTIN® plus TAXOTERE® (Docetaxel), PERJETA® plus HERCEPTIN® and TAXOTERE®, PERJETA® plus HERCEPTIN® or PERJETA® plus TAXOTERE®. Patients in the three drug group received preoperative therapy with PERJETA®, HERCEPTIN® and TAXOTERE® every 3 weeks for a total of 4 cycles and following surgery, all patients received 3 cycles of Fluorouracil, ELLENCE® (Epirubicin), and CYTOXAN® (Cyclophosphamide) – (FEC) IV every 3 weeks and HERCEPTIN® was continued every 3 weeks for a total of one year of therapy. The primary endpoint was pathological Complete Response (pCR) rate defined as the absence of invasive cancer in the breast. The FDA definition of pCR is the absence of invasive cancer in the breast and lymph nodes. All treatment groups were well balanced. Seven percent of patients had inflammatory breast cancer, 32% had locally advanced cancer and 70% had clinically node-positive breast cancer. Forty-seven percent of the patients had hormone receptor-positive disease. The FDA defined pCR rates were 39.3% in the PERJETA® plus HERCEPTIN® and TAXOTERE® group and 21.5% in the HERCEPTIN® plus TAXOTERE® group P=0.0063). Of Interest, the pCR rates in the three drug group were lower in patients with hormone receptor positive tumors compared to patients with hormone receptor negative tumors. The most common adverse events in the three drug group were alopecia, diarrhea, nausea and neutropenia. The accelerated approval by the FDA was based solely on the improved pCR rate with the three drug combination with no demonstrable improvement in event-free survival or overall survival. A confirmatory phase III trial is underway, with results expected in 2016. Gianni L, Pienkowski T, Im YH, et al. Lancet Oncol. 2012;13:25-32
PERJETA® (Pertuzumab) The FDA on September 30, 2013 approved PERJETA® for use in combination with HERCEPTIN® (Trastuzumab) and TAXOTERE® (Docetaxel) for the neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer. This combination was approved by the FDA in June 2012, for the treatment of patients with HER2-positive metastatic breast cancer
The FDA on September 30, 2013 approved PERJETA® for use in combination with HERCEPTIN® (Trastuzumab) and TAXOTERE® (Docetaxel) for the neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer. This combination was approved by the FDA in June 2012, for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease. PERJETA® is an injection and is a product of Genentech, Inc.
KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1):
The FDA on February 22, 2013 approved KADCYLA® for the treatment of patients with HER2-positive metastatic breast cancer who have received prior treatment with HERCEPTIN® (Trastuzumab) and a Taxane chemotherapy. KADCYLA® is marketed by Genentech U.S., Inc.
Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane.
SUMMARY: The HER or erbB family of receptors consist of HER1,HER2,HER3 and HER4. Overexpression of HER2 in breast cancer has been associated with higher risk for relapse as well as overall survival. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. It binds to the extracellular domain of the receptor and blocks the downstream cell signaling pathways. KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1) is an antibody-drug conjugate (ADC) comprised of the antibody Trastuzumab and the chemotherapy agent Emtansine, linked together. It inhibits HER2 signaling and destroys the HER2-positive tumor cells by delivering the chemotherapy agent Emtansine which binds to tubulin, directly inside the tumor cells. The EMILIA trial is a phase III study in which 991 patients with HER2-positive locally advanced or metastatic breast cancer who had previously received treatment with HERCEPTIN® and a Taxane chemotherapy, were enrolled. Patient received either KADCYLA® or XELODA® (Capecitabine) and TYKERB® (Lapatinib) doublet. The primary endpoints were Progression Free Survival (PFS), Overall Survival (OS) and safety. Patients receiving KADCYLA® had an improved PFS compared to XELODA® and TYKERB® (9.6 months vs 6.4 months, HR=0.65, P <0.0001). The median overall survival was 30.9 months in the KADCYLA® group and 25.1 months with the XELODA® and TYKERB® doublet. KADCYLA® is the fourth drug approved by the FDA, that targets the HER2 oncogene. The other FDA-approved drugs used to treat HER2-positive breast cancer include HERCEPTIN® (1998), TYKERB® (2007) and PERJETA® (Pertuzumab) (2012). Blackwell KL, Miles D, Gianni L, et al. J Clin Oncol 30, 2012 (suppl; abstr LBA1)
KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1) now approved
The FDA today approved KADCYLA® for the treatment of patients with HER2-positive metastatic breast cancer who have received prior treatment with HERCEPTIN® (Trastuzumab) and a taxane chemotherapy. In a large Phase III trial, KADCYLA® improved Progression Free Survival as well as Overall Survival compared to XELODA® (Capecitabine) and TYKERB® (Lapatinib). KADCYLA® is the fourth drug approved by the FDA, that targets the HER2 oncogene. The other FDA-approved drugs used to treat HER2-positive breast cancer include HERCEPTIN® (1998), TYKERB® (2007) and PERJETA® (Pertuzumab) (2012).
